Subscribe to Our Youtube Channel
Summary of Contents for Cyclomedica Technegas TechnegasPlus
- Page 1 TechnegasPlus Technegas® Generator USER MANUAL MNL-0007 Rev 07 EU-EN ENGLISH Manufactured in Australia by Cyclomedica Australia Pty Ltd...
-
Page 2: Preamble
The information found in this manual is the latest information at the time of delivery for the TechnegasPlus Technegas® Generator (TP). Model number 25000. Cyclomedica Australia Pty Ltd reserves the right to change the content of this manual without prior notice. You will be advised of any changes that may affect the use of your TechnegasPlus Technegas®... -
Page 3: Table Of Contents
Table of Contents Preamble ..........................2 Manufacturer’s Trademark and Copyright ..............2 Product Information ......................6 Device description ...................... 6 Intended use ....................... 6 Indications for use ...................... 6 Contraindications ......................6 Maximum exposure ....................7 Principle of operation ....................7 Operator responsibility ...................... - Page 4 10.2 Setting up the Argon gas supply ................23 10.3 Prepare the TP ......................23 10.4 Open drawer and remove Pulmotec® (Crucible) fragments ........24 10.5 Preparing the Pulmotec® (Crucible) ................ 25 10.6 Simmer Process ....................... 26 10.7 Multiple loadings ...................... 26 10.8 Burn Process ......................
- Page 5 17.4.4 Pulmotec® (Crucible) ..................48 17.4.5 Purge filter life ....................48 17.5 Identifying the Date of Manufacture ............... 48 17.6 Electromagnetic Compatibility ................49 18 End of Life decommissioning of the TP ................52 19 Further reading ........................ 53 MNL-0007 Rev 07 EU-EN 23 Nov 2022 TechnegasPlus Technegas®...
-
Page 6: Product Information
2 Product Information 2.1 Device description The TechnegasPlus Technegas® Generator (TP) is an electrically powered medical device for creating hydrophobic Technetium-99m labelled Carbon particles dispersed in Argon as an aerosol. Technegas® is the brand name for the system of medical devices and pharmaceuticals used in the production of the Technetium-99m radio-labelled Carbon aerosol also referred to as Technegas®. -
Page 7: Maximum Exposure
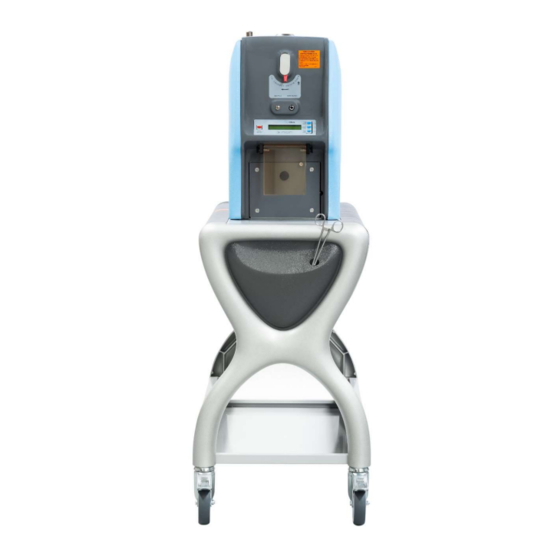
2.5 Maximum exposure The maximum exposure of the TP to the patient via ventilation through the PAS is 3 minutes in a 24-hour period. 2.6 Principle of operation Figure 1: TechnegasPlus Technegas® Generator (TP) The TP is a bespoke high temperature furnace; it is a Class IIb medical device. It uses a combination of graphite in the form of the Pulmotec®... - Page 8 Figure 2: Technegas® generation and delivery summary An Operator-controlled electrically operated valve in the chamber enables the patient to inhale Technegas® via the PAS which, by depositing on the surfaces of the alveoli of the lung, enables the functioning airways distribution to be mapped by the standard nuclear medicine technology, namely a gamma camera.
-
Page 9: Operator Responsibility
Informing Cyclomedica of complaints, mal-administrations, adverse events; Informing Cyclomedica requests for repair should the TP become faulty; and Any malfunction which results from the improper use, maintenance or service issues, improper repair, damage, alteration or modification by anyone other than a Cyclomedica approved service provider. -
Page 10: Precautions And Safety In Use
) of European or US Pharmacopoeia grade or equivalent, in the TP. Only the brass Technegas® Contacts, manufactured by Cyclomedica Australia, are validated to be used for the safe and effective performance of the TP and the production of Technegas®. The Technegas® Contacts are critical accessories for the consistent and safe performance of the TP. -
Page 11: Safety In Use
Technegas® inhalation. This clears Technegas® (the aerosol) from the delivery tubing and the patient's conducting airways. If a patient is unable to use a mouthpiece adequately, Cyclomedica recommends that a face mask is used with the PAS mouthpiece. Such face masks are available from Cyclomedica. -
Page 12: Warnings
Do not load the trolley with heavy items such as gas cylinders for transport. 4.4 Warnings Argon is an asphyxiation hazard. Always turn OFF the Argon supply when not connected to the TP. The internal components of the TP may be hot. ... -
Page 13: Operating Key Functions On The Tp
Issues with opening or closing of the Drawer may pose a radiation hazard if the TP has recently performed a Burn process. In this event, follow your site’s radiation safety plan and contact your Cyclomedica approved service provider. The Drawer must be closed when the TP is not in use. -
Page 14: Front And Rear Panel Descriptions
5.3 Front and Rear panel descriptions 1 – START button 2 – CLOSE button 3 – OPEN button 4 – Display 5 – Socket for Remote delivery button 6 – PAS connection behind metal cover 7 – Patient delivery button 8 –... -
Page 15: Installation
6 Installation 6.1 Installation information The installation for use must be performed by a Cyclomedica approved service provider. The approved service provider will complete the installation checklist to be signed by the Site Representative. 6.2 Location and storage Assign an area within the Nuclear Medicine department, preferably near the imaging room, for the production of Technegas®... -
Page 16: Connecting The Argon Gas
6.5 Connecting the Argon gas The TP is only designed for use with the supplied Argon hose and the Cyclomedica Argon low-pressure regulator. Figure 6(a, b): Connecting the Argon supply hose To connect the Argon hose to the gas Argon inlet of the TP, press the black cover, insert the connector into the socket, and pull the black cover back to engage the locking mechanism. -
Page 17: Battery Charging
Sodium Pertechnetate Tc 6.8 External Transportation Prior to moving the TP from or between facilities, Cyclomedica approved service provider is required to prepare the TP for safe external transport. Contact your Cyclomedica approved service provider or Cyclomedica for guidance/assistance. -
Page 18: Patient Preparation
7 Patient Preparation Figure 7: Supine administration of Technegas® For the administration of Technegas® to the patient, Operators should follow the details in the Information for Use for the Technegas® PAS and the Patient Information Leaflet for the Pulmotec® (Crucible). Technegas®... - Page 19 1. Select the appropriate mouthpiece and attach it to the T- section connector at one end of the PAS (end closest to filter). 2. Place the mouthpiece in the patient’s mouth ensuring a total seal by the patient’s lips. 3. Place a nose-clip on the patient’s nose. 4.
-
Page 20: Dosimetry
8 Dosimetry Refer to the Pulmotec® (Crucible) Patient Information Leaflet for more information regarding dosimetry. The recommended activity to be loaded in the Pulmotec® (Crucible) is between 250 - 700 MBq (7mCi – 19mCi) of Tc sodium pertechnetate. The recommended radioactive concentration for the Pulmotec® (Crucible) 130µL is ... -
Page 21: Technegas® Ventilation Technique
9 Technegas® ventilation technique The recommended technique for most patients is normal tidal breathing without breath- holding. Instruct the patient to maintain normal tidal breathing. When the patient ends exhalation, DEPRESS the delivery button, to allow the delivery of Technegas® from the TP to the patient’s lungs. ... -
Page 22: Production Of Technegas® Prior To Administration
10 Production of Technegas® prior to administration 10.1 Safety and Quality check 1. Wear gloves and use Good Radiation Practice. 2. Inspect the Pulmotec® (Crucible) for visible damage or defects. 3. Visually check that the ethanol used for wetting the Pulmotec® (Crucible) is ≥ 95% pure, within expiry and non-denatured. -
Page 23: Setting Up The Argon Gas Supply
TP. 3. If necessary, switch ON the TP. The display will show the name Cyclomedica Australia Pty Ltd, the current TP software version and the date and time. If a ‘purge’ has not been carried out since the last Technegas® (aerosol) generation, the TP will then check if the Drawer is closed and perform a purge operation. -
Page 24: Open Drawer And Remove Pulmotec® (Crucible) Fragments
10.4 Open drawer and remove Pulmotec® (Crucible) fragments During Technegas® generation all internal components of the chamber and gas pathway leaving the chamber are radioactively contaminated. Use Good Radiation Practices including wearing disposable gloves. The internal components of the TP may be HOT! 1. -
Page 25: Preparing The Pulmotec® (Crucible)
10.5 Preparing the Pulmotec® (Crucible) Figure 11 (a, b): Installing the Pulmotec® (Crucible) into the TP Drawer 1. Using the forceps, pick up the Pulmotec® (Crucible) from its packaging and place it on a non-contaminated flat surface. 2. To prime the Pulmotec® (Crucible), use a 1mL syringe filled with 95% pure non- denatured ethanol to fill the Crucible cavity only, then draw the ethanol back into the syringe and discard. -
Page 26: Simmer Process
Do not overfill the Pulmotec® (Crucible), the meniscus should be concave. USE ONLY a solution of sodium pertechnetate ( Tc) of European or US Pharmacopoeia grade or equivalent in the TP 10. To close the Drawer, PRESS and HOLD the DRAWER INTERLOCK knob, then PRESS and HOLD the ‘CLOSE’... -
Page 27: Burn Process
10.8 Burn Process 1. Press the START button to initiate the Burn process. 2. When the burn is complete the display will read: BURN VERIFIED 3. The display will then change to: DISCONNECT THE MAINS PLEASE Technegas® is now prepared and ready for inhalation by the patient within 10 minutes. -
Page 28: Administration Of Technegas
11 Administration of Technegas® Use disposable gloves and conduct Good Radiation Practice. Do not press the Patient Delivery Button or Remote Delivery Button until the patient is being ventilated through the TP as Technegas® will be released. The inhaled dose for an adult is usually about 20-50MBq (0.54-1.35mCi). Refer to the Pulmotec®... -
Page 29: After Administration To The Patient
If the TP does not perform the automated Purge process after the Burn process, contact your Cyclomedica approved service provider. Do not leave the TP unattended while the Argon supply is turned ON. Ensure the Drawer is left closed and the Argon supply is OFF when the TP is not in use. -
Page 30: Operating The Simmer Plate
13 Operating the Simmer Plate Figure 14: Simmer Plate set up The Drawer contains a Simmer Plate that allows simultaneous preparation of up to five Pulmotec® (Crucibles) with multiple loadings of Tc sodium pertechnetate. The Simmer Plate is useful for sites performing multiple Technegas® operations in a ... - Page 31 Figure 15: Loading the Simmer Plate 5. Using a 1mL syringe with needle, fill the Pulmotec® (Crucible) with 250 - 700 MBq (7mCi – 19mCi) of Tc sodium pertechnetate. The meniscus should be concave or flat. 6. Close the Simmer Plate lid using the forceps. When ready to perform the production of Technegas®, carefully place the Pulmotec®...
-
Page 32: Disposal Of Contaminated Items
The disposal of radioactive and infectious waste is subject to the regulations and the appropriate licenses of the local Competent Authority or Regulatory Body. If advice on disposal is required, Cyclomedica recommends that Operators contact their local Competent Authority or Regulatory Body. -
Page 33: Maintenance
15 Maintenance The TP must not be serviced or maintenance activities conducted during clinical use with a patient. 15.1 Operator Maintenance The Operator must: Replace the Technegas® Contacts every 50 burns as described in 15.3 C HANGING ® C ECHNEGAS ONTACTS Empty the Ash tray of Pulmotec®... -
Page 34: Changing The Brass Technegas® Contacts
15.3 Changing the Brass Technegas® Contacts The TP automatically counts down the remaining Burns for each new set of Technegas® Contacts installed. The Technegas® Contacts must be replaced every 50 Burns to ensure the safe and efficacious production of Technegas®. ... - Page 35 Do not over-tighten the screws as excessive force may damage the screw thread inside the brass pedestal. 8. Switch ON the TP and the following message will be displayed: "CONTACTS [OPEN ] = NO " "CHANGED ?? [CLOSE] = YES" 9.
-
Page 36: Display Language Selection
15.4 Display Language Selection The user interface language of the TP may be selected at installation or during a general service by the approved service provider. The default language of the TP is English. The other available languages are: French ... -
Page 37: Authorised Service & Maintenance
If a fault arises in the operation of the TP, switch it OFF and call your local distributor or Cyclomedica for service. Do not operate the TP until the fault is resolved. Please contact your Cyclomedica approved service provider to request a service for your TP. -
Page 38: Troubleshooting
16 Troubleshooting 16.1 Troubleshooting Table Error Message/Fault Meaning and Recommended Actions LOW ACTIVITY IN INHALED DOSE If the patient has a low inhaled dose or low activity count rate when imaged, this may be due to the TP producing a low yield of Technegas®. - Page 39 Error Message/Fault Meaning and Recommended Actions CRUCIBLE FAILED TO REACH FULL Indicates that temperature was incorrect TEMPERATURE during burn stage. Check crucible is not cracked. When inserting a new crucible rotate it back and forth a few times. Check contacts for possible Carbon ...
- Page 40 Where possible, re-set the circuit breaker or replace the fuse. If the unit trips again, contact your Cyclomedica approved service provider. ***If unit is still in error, contact your approved service provider for assistance*** MNL-0007 Rev 07 EU-EN 23 Nov 2022 TechnegasPlus Technegas®...
-
Page 41: Lcd Messages
16.2 LCD messages LCD MESSAGE Meaning and Actions required The TP is purging (cleaning) the chamber prior to allowing WAIT PURGING CHAMBER the Drawer to be opened. The Drawer is ready to be opened to load a Pulmotec® OPEN DRAWER TO (Crucible). - Page 42 LCD MESSAGE Meaning and Actions required Indicates to the Operator to press the START button to [START] RELEASES THE unlock the Patient Delivery Valve. GAS VALVE If the CLOSE button is pressed without first pressing the PRESS DRAWER DRAWER INTERLOCK knob this message will be INTERLOCK KNOB displayed along with an audible alert.
- Page 43 LCD MESSAGE Meaning and Actions required Indicates that there may be an issue with the Drawer. Try THE DRAWER FAILED TO closing and opening the Drawer again. If this fails to fix OPEN IN THE TIME the issue, then contact your approved service provider for ALLOWED assistance.
- Page 44 LCD MESSAGE Meaning and Actions required Indicates that a failure has occurred in controlling the TRIAC FAILURE Pulmotec® (Crucible) temperature. Contact your approved service provider for assistance. Indicates that the TP has detected a failure and requires TURN OFF AND TRY the power to be switched OFF and then back ON again to AGAIN reset the TP.
- Page 45 LCD MESSAGE Meaning and Actions required The TP detects that its internal temperature is too high. CASE TEMPERATURE TOO Wait until the TP cools down to an allowable HIGH WAIT temperature. No more than two Burns operations per hour is specified. MNL-0007 Rev 07 EU-EN 23 Nov 2022 TechnegasPlus Technegas®...
-
Page 46: Label And Symbols
16.3 Label and Symbols CE marking is a declaration by the Product code manufacturer that the product meets all the essential health and safety requirements in the appropriate provisions of the relevant legislation (in this case Medical Device Regulations) implementing particular European Directives. -
Page 47: Specifications
17 Specifications Further information may be found in the Cyclomedica TechnegasPlus Technegas® Generator Service Manual. 17.1 General Supply Voltage 200-240 VAC +/- 5% Transformer tapping set at installation. Supply Frequency 50-60 Hz Mains Current Steady State < 0.2 A RMS Mains Current Maximum –... -
Page 48: Consumables
Argon Gas must be at least high purity grade (≥99.997%) (Grade 4.7 or greater). 17.4.2 Technegas® PAS The Technegas® PAS bespoke for the TP and is available from your Cyclomedica approved service provider or your local distributor. ... -
Page 49: Electromagnetic Compatibility
17.6 Electromagnetic Compatibility Guidance and manufacturer’s declaration – electromagnetic emissions The TP is intended for use in the electromagnetic environment specified below. The User of the TP should assure that it is used in such an environment. Emissions Tests Compliance Electromagnetic Environment - Guidance The TP uses RF energy only for its internal RF Emissions... - Page 50 Guidance and manufacturer’s declaration – electromagnetic immunity The TP is intended for use in the electromagnetic environment specified below. The User of the TP should assure that it is used in such an environment. Immunity test IEC 60601 Compliance level Electromagnetic Test level environment - guidance...
- Page 51 Recommended separation distances between portable and mobile RF communications equipment and the TP The TP is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The user of the TP can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the TP as recommended below, according to the maximum output power of the communications equipment.
-
Page 52: End Of Life Decommissioning Of The Tp
The TP has a recommended use life of 10 years from date of manufacture. You must contact your Cyclomedica approved service provider for assistance with the decommissioning of the TP. Decommissioning of the TP may be subject to local regulations for medical devices. -
Page 53: Further Reading
Leblanc M, Tessier M, Ollenberger and O’Brien Christopher. CANM guidelines for ventilation/perfusion (V/P SPECT) (2018); https://canm-acmn.ca/guidelines A full discussion of the Technegas® technology, clinical references and highlights may be found at: http://www.cyclomedica.com/clinical MNL-0007 Rev 07 EU-EN 23 Nov 2022 TechnegasPlus Technegas® Generator User Manual 53 of 53...



Need help?
Do you have a question about the Technegas TechnegasPlus and is the answer not in the manual?
Questions and answers