Summary of Contents for Vitalograph Asma-1
- Page 1 25/08/2017 07380_9 Asma-1 Modelo 4000 Manual de treinamento do usuário file:///C:/Users/Dynamic%20Health/Downloads/Vitalograph%20asma-1%20-%20User%20Training%20Manual.html 1/21...
- Page 2 Vitalograph (Irl.) Ltd. Gort Road Business Park, Ennis, Co. Clare, Ireland Phone: (065) 6864100 Fax: (065) 6829289 e-mail: sales@vitalograph.ie www.vitalograph.ie © Copyright Vitalograph 2013 Current Edition (Issue 9) Cat. No. 07380 is a registered trademark file:///C:/Users/Dynamic%20Health/Downloads/Vitalograph%20asma-1%20-%20User%20Training%20Manual.html 2/21...
-
Page 3: Table Of Contents
4. REVIEWING PREVIOUS RESULTS 5. DELETING ALL RESULTS HISTORY 7. CLEANING INSTRUCTIONS 1. HOME USE CLEANING AND DISINFECTION OF THE VITALOGRAPH ASMA-1 2. CLEANING AND DISINFECTING THE VITALOGRAPH ASMA-1 IN CLINIC USE 1. Table of Materials Used & Cleaning/Disinfection Methods 2. - Page 4 25/08/2017 07380_9 file:///C:/Users/Dynamic%20Health/Downloads/Vitalograph%20asma-1%20-%20User%20Training%20Manual.html 4/21...
-
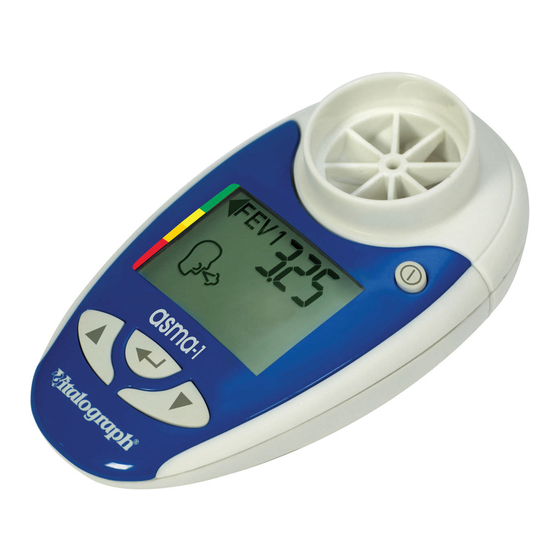
Page 5: Description Of The Vitalograph Asma-1
Many people over the ages of five will benefit from monitoring their asthma with the Vitalograph asma-1, indicating when and how much to use their reliever medication. It will also help the doctor because scores make it easier to see how well the asthma is controlled and when treatment needs changing. -
Page 6: Features Of The Vitalograph Asma-1
2. Insert the mouthpiece and turn on the device. 5. POWER MANAGEMENT IN THE VITALOGRAPH ASMA-1 The asma-1 operates with 2 AAA 1.5V disposable batteries. If the battery symbol flashes the batteries need to be replaced. Replace the batteries by removing the battery door on the underside of the device. -
Page 7: Setting Management Zones
Note: to de-activate zones, set both the PEF and FEV1 reference values to 000/0.00. 6.2. Setting Management Zones The Vitalograph asma-1 can be set for use with 3 or 4 zone management plans. The zone percentages are factory set to 2 boundaries, 80% & 50%, i.e. 3 Zones (80-100%, 50-80%, 0-50%). -
Page 8: How To Use The Vitalograph Asma-1
If the subject experiences dizziness or fatigue during the test session, wait until this passes before blowing again or terminate the session. 6.4. Reviewing Previous Results The Vitalograph asma-1 can store up to 600 test sessions. In order to view previously performed test sessions, follow these steps: 1. Turn the device on, . -
Page 9: Cleaning Instructions
7. CLEANING INSTRUCTIONS 7.1. Home use cleaning and disinfection of the Vitalograph asma-1 The Vitalograph asma-1 should continue to give reliable measurements for up to three years in home use. Then replace it with a new device. Keep it clean and dust free. If you suspect the device is damaged or is measuring incorrectly, contact the doctor immediately. -
Page 10: Removing The Flowhead For Cleaning And Disinfecting
Maximum of 50 cleaning cycles allowed. All external parts of the Vitalograph asma-1 require cleaning, i.e. the removal of visible particulate contamination. The parts of the device that make up the flowhead, which comes into contact with the breath of the subjects being tested, also require disinfecting. -
Page 11: Fault Finding Guide
Cleaning of Medical Equipment: Guidance on Decontamination from the Microbiology Committee to Department of Health Medical Devices Directorate, 1996” Recommendations for chemical disinfectants are derived from the PHLS publication “Chemical Disinfection in Hospitals 1993" 8. FAULT FINDING GUIDE file:///C:/Users/Dynamic%20Health/Downloads/Vitalograph%20asma-1%20-%20User%20Training%20Manual.html 11/21... -
Page 12: Customer Service
Service and repairs should be carried out only by the manufacturer, the approved importer or by Service Agents specifically approved by Vitalograph. For the names and addresses of approved Vitalograph Service Agents or to arrange spirometry workshops, please refer to the contact information at the start of this manual. -
Page 13: Technical Specifications
Battery status Battery status Full Battery status Half Battery status Quarter Battery status Empty (flashing) Blow Now Symbol Bad Test Symbol (Slow start or Cough) Memory 90% - 100% Full Icon Flashes when memory reaches 100% 12. TECHNICAL SPECIFICATIONS file:///C:/Users/Dynamic%20Health/Downloads/Vitalograph%20asma-1%20-%20User%20Training%20Manual.html 13/21... - Page 14 Auto power down time 2 minutes Note: 1. All values displayed by the Vitalograph asma-1 are expressed as BTPS values. 2. Take care not to block the mouthpiece with the tongue or teeth. A ‘spitting’ action or coughing will give false readings.
-
Page 15: Ce Notice
Marking by the symbol indicates compliance of the Vitalograph asma-1 to the Medical Devices Directive of the European Community. Such marking is indicative that the Vitalograph asma-1 meets or exceeds the following technical standards: Guidance and manufacturer’s declaration – electromagnetic emissions The 4000 Respiratory Monitor asma-1 is intended for use in the electromagnetic environment specified below. - Page 16 07380_9 Guidance and manufacturer’s declaration – electromagnetic immunity The 4000 Respiratory Monitor asma-1 is intended for use in the electromagnetic environment specified below. The customer or the user of the 4000 Respiratory Monitor asma-1 should assure that it is used in such an environment.
- Page 17 Guidance and manufacturer’s declaration – electromagnetic immunity The 4000 Respiratory Monitor asma-1 is intended for use in the electromagnetic environment specified below. The customer or the user of the 4000 Respiratory Monitor asma-1 should assure that it is used in such an environment.
- Page 18 Recommended separation distances between portable and mobile RF communication equipment and the 4000 Respiratory Monitor asma-1 The 4000 Respiratory Monitor asma-1 is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the 4000...
-
Page 19: Fda Notice
25/08/2017 07380_9 14. FDA NOTICE Caution: Federal Law restricts this device to sale by, or on the order of a physician. file:///C:/Users/Dynamic%20Health/Downloads/Vitalograph%20asma-1%20-%20User%20Training%20Manual.html 19/21... -
Page 20: Declaration Of Conformity
15. DECLARATION OF CONFORMITY Product: 4000 Respiratory Monitor asma-1 Vitalograph hereby ensures and declares that the above product associated with this user manual, is designed and manufactured in accordance with the following QMS regulations and standards: European Medical Devices Directive {MDD} 93/42/EEC, as amended. -
Page 21: Guarantee
07380_9 16. GUARANTEE Subject to the conditions listed below, Vitalograph Ltd. and its associated companies, (hereinafter called the Company) guarantee to repair or at its opinion replace any component thereof, which, in the opinion of the Company is faulty or below standard as a result of inferior workmanship or materials.

















Need help?
Do you have a question about the Asma-1 and is the answer not in the manual?
Questions and answers