Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for SurgEase LumenEye X1
- Page 1 LumenEye ® Instructions for Use...
-
Page 2: Table Of Contents
Contents CE Markings LumenEye X1 system ® LumenEye X1 Medical Device ® Intended Use Contraindications & Warnings Caution Principle of Operation Symbols Docking Case - Getting Started Docking Case - Cleaning, Disinfection and Maintenance LumenEye X1 - Assembly & Use ®... -
Page 3: Ce Markings
Medical Device Regulation 2017/745. Copyright 2020 SurgEase Innovations Ltd referred to in this document as ‘SurgEase’. All rights are reserved. No one is permitted to reproduce or duplicate, in any form, this manual or any part thereof without the explicit permission of SurgEase. -
Page 4: Lumeneye X1 System
2. LumenEye X1 system ® LumenEye X1 system Components ® LumenEye X1 portable docking case ® 2 Storage for LumenEye X1 consumables and tablet charger ® 3 LumenEye X1 storage recess ® 4 LumenEye X1 cable-tidy recess ® 5 LumenEye X1 device mount ®... -
Page 5: Lumeneye ® X1 Medical Device
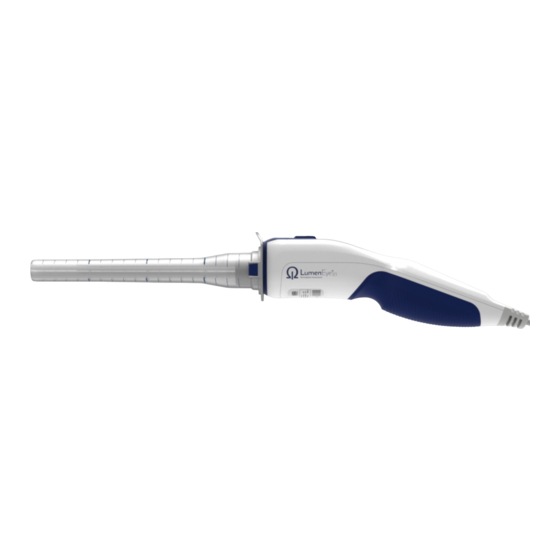
3. LumenEye X1 Medical Device ® LumenEye X1 Medical Device Parts List ® 1 LumenEye X1 handheld endoscope ® 1.1 Camera and LEDs 1.2 Stainless steel camera tube 1.3 Air barb 1.4 Compliance label 1.5 Insufflation bellows 1.6 USB connector 2 Sheath (single-use, applied part) 3 Obturator (single-use) 4 Manifold (single-use) -
Page 6: Intended Use
These components must not be re-used and be discarded in clinical waste once a procedure is completed. Components must only be purchased from SurgEase or an approved distributor. Do not attempt to use the LumenEye ®... -
Page 7: Contraindications & Warnings
5. Contraindications & Warnings Using the LumenEye X1 in patients with BSE/TSE, HIV, Hepatitis C or other ® communicable pathogens transmittable through direct physical contact should be undertaken in consideration of local standard operating procedures on infection control. Please see section 12 & 13 for further details on cleaning and disinfection. -
Page 8: Caution
In compliance with the European Directive on Waste Electrical and Electronic Equipment (WEEE) 2002/96/EC, do not dispose of this product as unsorted municipal waste. This device contains WEEE materials; please contact SurgEase regarding return or recycling of the LumenEye ®... -
Page 9: Symbols
The intended users of the LumenEye X1 should be trained healthcare ® professionals. Users of the system are expected to have a pre-requisite level of medical knowledge and experience in order to review and analyse the captured data. 8. Symbols Indicates name and address of the legal manufacturer Prefixes date of manufacture The product is a medical device as defined by Medical Device... -
Page 10: Docking Case - Getting Started
9. Docking Case - Getting Started Setting Up Ensure the docking case is placed on Open the docking case lid to a fully a solid, flat and clean surface before retracted position and remove all opening. packaging. POWER BUTTON HERE Tablet Feature List Open the tablet screen to preferred angle (1), connect the LumenEye... -
Page 11: Docking Case - Cleaning, Disinfection And Maintenance
Storage DO NOT OBSTRUCT To prevent unnecessary damage, ensure Ensure that the tablet is fully switched off and the tablet screen is placed in the ® the LumenEye X1 is packed in the closed position before shutting the case lid. dedicated storage recess and the cable is Ensure there is no obstruction of the mount wrapped in the cable-tidy recess as shown. -
Page 12: Lumeneye ® X1 - Assembly & Use
11. LumenEye X1 - Assembly & Use ® TOP VIEW Perform a visual inspection to ensure the The patient should preferably be examined device is clean. Check for visible cracks or in the left lateral position. physical damage. Connect the camera to the tablet, check all LEDs are illuminating sufficiently and confirm the camera feed is working. - Page 13 CLICK! Push the obturator fully into the sheath then Once inserted, remove the obturator carefully insert into the patient. Please note whilst leaving the sheath inside the patient that the arrow on the sheath and obturator and insert the LumenEye ®...
-
Page 14: Lumeneye ® X1 - Cleaning & Disinfection: Preparation
Cleaning Notes: Do not re-use cloths or wipes. Soap, detergents or enzymatic cleaners should be used in accordance with the manufacturer’s instructions. SurgEase is not responsible for damage incurred during the cleaning process with products where no material compatibility evaluation has been performed. -
Page 15: Lumeneye ® X1 - Cleaning & Disinfection: Instructions
13. LumenEye X1 - Cleaning & Disinfection: Instructions ® After use, remove and discard the sheath After each examination, ensure all lubricant gel is wiped completely from the camera ® and manifold from the LumenEye tip. The device should not be left soaking Single use parts should be disposed as in gel. - Page 16 Wrap wipe Manifold around port area Q Tip Remove a Tristel Pre-Clean Wipe from the Thoroughly wipe the indentations in the sachet and unfold it in the palm of your manifold port. Wrap the wipe around a hand. Fully wipe the device including Q Tip and use this to reach every corner.
-
Page 17: Charging Instructions
Fully wipe the device including cable with Use a lint-free soft dry cloth to thoroughly the Tristel Rinse Wipe. Ensure all surfaces are dry the LumenEye ® wetted once and foam and other residues from the previous steps are removed. Perform a visual inspection of the device ®... -
Page 18: Software Compatibility
This warranty is also void if the instrument is not used in accordance with the manufacturer’s recommendations or if it is repaired by any agent other than SurgEase or an authorised agent. First-use date determines warranty requirements. No other express warranty is given. -
Page 19: Disposal
Improper handling of waste may negatively impact the environment and human health. Full co-operation with appropriate disposal advice improves the use of natural resources. For more information about recycling this product, contact SurgEase. IFU-101-1.0... -
Page 20: Troubleshooting
19. Troubleshooting Condition Description and Corrective Action Interrupted Feed If the video feed is lost or frozen, ensure that the USB connector is securely in the correct port. If this issue continues, ensure that there are no other electronic devices in proximity that could cause interference. -
Page 21: Environmental Information
20. Environmental Information Condition Temperature Relative Atmospheric Pressure Range Humidity Operating +10 °C to +40 °C 30% to 75% 700 hPa to 1060 hPa (+50 °F to +104 °F) (non-condensing) Storage and +10 °C to +40 °C 20% to 95% 700 hPa to 1060 hPa (+50 °F to +104 °F) (non-condensing) - Page 22 INDICATION FOR USE LumenEye X1 is indicated for use in diagnostic and interventional endoscopic procedures to provide illumination ® and visualisation of the anorectum. Should only be used by suitably trained healthcare professionals. IFU-101-1.0...
Need help?
Do you have a question about the LumenEye X1 and is the answer not in the manual?
Questions and answers