Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Deta-Elis DETA Ritm-13
- Page 1 ELIS Research and Development Enterprise Electromagnetic Field Treatment Device DETA Ritm-13 Passport (13 treatment programs + 3 automatic programs) The latest scientifi c opinions on the fi ght against disease A unique medical procedure M I S S I O N...
- Page 2 © ELIS Research & Development Enterprise, 2010 All rights reserved. Partial or complete photomechanical reproduc- tion and recording onto electronic media is prohibited. Printed in Moscow.
-
Page 3: Table Of Contents
Contents 1. Introduction 2. Purpose 3. Main specifi cations of the device 4. Package contents 5. Information about the device 6. Getting started 7. Operation 8. Directions for use of the device 9. Contraindications to use 10. Storage 11. Transportation 12. -
Page 4: Introduction
1. Introduction Treatment device “DETA-RITM-13” TУ 9444-001-27970873-2006 (hereinafter referred to as the device) is designed for electro- magnetic therapy. The device is designed for individual use: • In clinical practice by doctors of various specializations. • Outpatient use. • Home use. Certifi... -
Page 5: Purpose
2. Purpose Treatment device “DETA-RITM-13” is designed for low-fre- quency non-contact electromagnetic therapy. The device is used to prevent and treat various conditions. Treatment is carried out via the eff ects of low-frequency electromagnetic radiation on the body in a physiological range of 0.1 to 100 Hz, and allows you to conduct therapy for a wide range of diseases with programs especially developed for this. -
Page 6: Main Specifi Cations Of The Device
3. Main specifi cations of the device 3.1. Basic parameters and dimensions 3.1.1. The device requires 2 x AA batteries (R6UPR) 3.1.2. Total dimensions of the device: not more than 111x70x25 mm. 3.1.3. Device weight: not more than 0.15 kg. 3.2. -
Page 7: Information About The Device
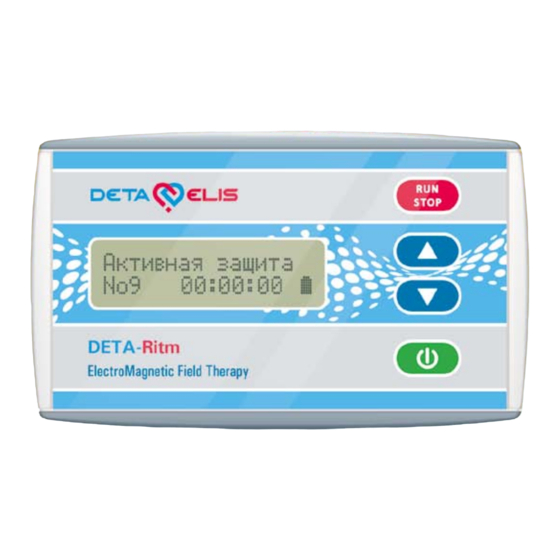
5. Information about the device а Fig. 1 Fig. 2 Figure 1 - Front Panel: • Power button • Arrows to alternate between programs • Button to start and stop programs • Screen (switched on). Fig. 2 - Side Panel. The upper side of the device has two sockets: a) a round socket for external power supply (not supplied;... -
Page 8: Getting Started
6. Getting started 6.1. Insert the batteries into the battery compartment, observ- ing correct polarity. The device will now start and perform a test for functionality. The appearance of a message on the dis- play (see Fig. 1) and audible signal indicates the functionality of the device. -
Page 9: Operation
7. Operation 7.1. Switch on the device, by pressing and holding it for 3 seconds (protection against accidental operation) before mov- ing to the treatment program selection mode. A single audible signal should sound. The display will read: the name of the pro- gram in the top row, and the bottom line will contain the num- ber of a program, the program time in hours:minutes:seconds, and the battery indicator. -
Page 10: Directions For Use Of The Device
8. Directions for use of the device Point of eff ect. The device has a mild, harmless eff ect on the body, and can be used for both adults and children with no age restrictions. Therapy is carried out by a course of treatment consisting of individual sessions. -
Page 11: Contraindications To Use
9. Contraindications to use Contraindications to the use of “DETA-RITM-13” include: • pregnancy; • the presence of a transplanted organ (kidney, etc.); • decompensated heart disease. 10. Storage The device without packing must be kept indoors at tem- peratures between 10 to 35°C and a relative humidity of not more than 80%. - Page 12 During the warranty period, the owner is entitled to free re- pairs on presentation of a warranty repair coupon. Warranty repairs are performed on the territory of the manufacturer. Transportation of the faulty device is at the buyer’s expense. Without presentation of a warranty repair coupon and test certifi...
- Page 13 Certifi cate of Acceptance “DETA-RITM-13” device Serial No. produced and accepted in accordance with mandatory require- ments of state standards and technical documentation in eff ect and established as fi t for use Technical control mark: seal (signature) (name) (date of issue)
- Page 15 ELIS Research & Development Enterprise LLC 4 offi ce 2408, Savelkinskiy Pr., Zelenograd Moscow Russia, 124482 Tel.: +7 (495) 981-91-60, +7 (495) 981-91-62 Coupon No. 1 for warranty repairs to the device “DETA-RITM-13” Serial No. Sale shop (name of trading organization) Shop seal (signature) Owner’s name and address...
- Page 17 ELIS Research & Development Enterprise LLC 4 offi ce 2408, Savelkinskiy Pr., Zelenograd Moscow Russia, 124482 Tel.: +7 (495) 981-91-60, +7 (495) 981-91-62 Coupon No. 2 for warranty repairs to the device “DETA-RITM-13” Serial No. Sale shop (name of trading organization) Shop seal (signature) Owner’s name and address...
- Page 19 ELIS Research & Development Enterprise LLC 4 offi ce 2408, Savelkinskiy Pr., Zelenograd Moscow Russia, 124482 Tel.: +7 (495) 981-91-60, +7 (495) 981-91-62 Coupon No. 3 for warranty repairs to the device “DETA-RITM-13” Serial No. Sale shop (name of trading organization) Shop seal (signature) Owner’s name and address...
- Page 20 4 offi ce 2408, Savelkinskiy Pr., Zelenograd, Moscow, Russia, 124482 4 office 2408, Savelkinskiy Pr., Zelenograd, Moscow, Russia, 124482 Tel.: +7 (495) 981-91-62, +7 (495) 981-91-60 Tel.: +7 (495) 981-91-62, +7 (495) 981-91-60 e-mail: elis@deta-elis.ru e-mail: elis@deta-elis.ru www.deta-elis.ru www. deta-elis.ru...



Need help?
Do you have a question about the DETA Ritm-13 and is the answer not in the manual?
Questions and answers
Какой программатор нужно для прибора дета-ритм-13
The Deta-Elis DETA Ritm-13 device is programmed with a computer. No specific programmer is mentioned.
This answer is automatically generated