Summary of Contents for Mobidiag Novodiag NVD-INST-B
- Page 1 Novodiag® System NVD-INST-B NVD-DSW For in vitro diagnostic use For use with Novodiag® Tests User Manual English Version 4-0 Issued in April 2020...
-
Page 3: Table Of Contents
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Table of contents About this manual ....................................1 1.1. Aim of the user manual ................................1 1.2. Conventions used in this manual............................1 1.3. Glossary of terms and abbreviations ........................... 1 1.4. - Page 4 Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 6.4. Starting a run ..................................... 25 6.5. Shutting down the Novodiag® System ..........................29 Preventive maintenance and controls ............................29 7.1. Preventive maintenance ............................... 29 7.2. Controls ......................................29 Decontamination and disposal ..............................30 8.1.
-
Page 5: About This Manual
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 About this manual 1.1. Aim of the user manual This user manual is intended solely for the users of the Novodiag® System, including the Novodiag® Instrument, Novodiag® Software and Novodiag® Test Cartridge, and the Touchscreen Computer and additional components provided with the system. -
Page 6: Glossary Of Symbols
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Real-time PCR Real-time Polymerase Chain Reaction. A method for detecting an amplified PCR product based on incorporation of a fluorescent allowing monitoring of PCR product. Targeted approach Diagnostic testing for a single target. Syndromic approach Diagnostic testing based on symptoms and allowing a large panel of pathogen screening in one single test. -
Page 7: Overview Of The Novodiag® System
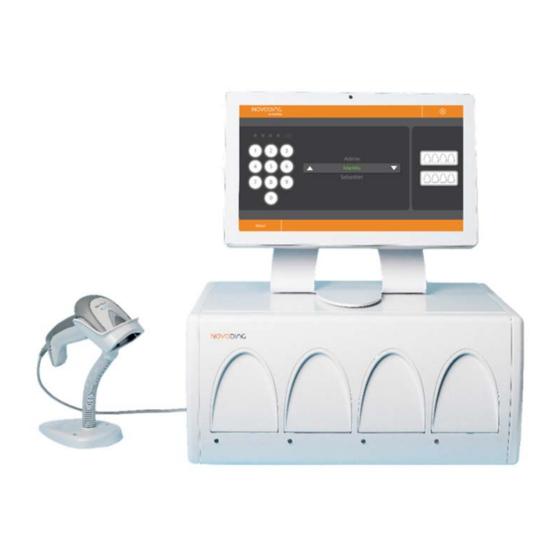
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Overview of the Novodiag® System 2.1. Intended purpose 2.1.1. Novodiag® Instrument Novodiag® Instrument is an in vitro diagnostic medical device intended to be used in combination with Novodiag® Software and Novodiag® Test specific cartridges to detect nucleic acid targets contained in clinical specimens. Novodiag®... - Page 8 Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Novodiag® Post (ref. NVD-TSCP-A) or Novodiag® Arm (ref. NVD-TSCA-A). The Novodiag® Instrument, the Touchscreen Computer and the Barcode Scanner are delivered with the necessary power and connection cables. The Novodiag® Test Cartridges are provided separately. 2.4.1.
- Page 9 Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 2.4.2. Touchscreen Computer (ref. NVD-TSC-A) The Touchscreen Computer is supplied with the Novodiag® Instrument. It runs the Novodiag® Software and provides the necessary connection to the Novodiag® Instrument, external network and Barcode Scanner. It is operated by Microsoft Windows 10 operating system.
-
Page 10: Principle Of Operation
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Figure 4. Novodiag® Test Cartridge description (before removing the peelable protective cover) After removing the peelable protective cover, the sample is introduced in the sample tank and the cartridge is closed by a cap supplied with the Novodiag® Test Cartridge (see Figure 5). Figure 5. -
Page 11: Principles Of Fluidic Operations
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 3.1. Principles of fluidic operations The distribution of fluids in the Novodiag® Test Cartridge from tank to tank and to the various processing chambers are performed by positive and negative pressure operated by an integrated syringe and directed by the activation of integrated valves by the Novodiag®... -
Page 12: Installation Requirements
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 4.1.2. For Novodiag® Test Cartridges Refer to the Novodiag® Test specific Instructions for Use. 4.2. Installation requirements Table 3. Requirements for each installation configuration Space requirement Weight Max power Number of (W x D x H) requirement consumption (W) -
Page 13: Packaging Content
The Touchscreen Computer, the Barcode Scanner and the Post or Arm are delivered in separate packages. CAUTION The Novodiag® Instrument unpacking must be performed by Mobidiag representative only. CAUTION Transport cartridge are only used to prevent any damage during shipment. - Page 14 NOTE Always remove Novodiag® Test Cartridges prior to shutting down the Novodiag® Instrument. CAUTION In a stack conformation (from 2 to 4 instruments), Novodiag® Instruments are interconnected. Do not shut down a Novodiag® Instrument separately, you risk impairing the whole stack functioning. Contact Mobidiag representative for assistance.
- Page 15 Novodiag® Software operation. • Do not install any software other than the Novodiag® Software. If your institution requires it, please consult with Mobidiag representative. Do not modify or erase any file from the Novodiag® Software folder. •...
-
Page 16: Novodiag® Software
Table 5. See chapter 5.6.2 (user management) for details on how to create users and modify their permissions. Mobidiag retains one admin user account for service personnel to use. Table 5. User credentials... -
Page 17: Home View
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 NOTE The Novodiag® Software is navigated in the same manner as any typical touch device. Most actions are performed by tapping, some require scrolling. No double tap is required. Navigation is from left to right. The Novodiag®... - Page 18 Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Table 6. Home view symbols Stack view symbols Instrument/Slot status Instrument displayed on the instrument view Instrument in stack not displayed on instrument view Instrument not connected Instrument disabled Test creation: Slot empty, ready for run Test creation: Slot selected, next run will be created to this slot Test creation: Run created, ready to start Slot initialising...
-
Page 19: Starting A Run
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Failed Run Slot disabled Instrument view In the Instrument view, each slot is represented with a pane. In this pane, a circular progress symbol indicates the slot status. More information is indicated by the symbols inside the circle, as explained in Table 7. Table 7. -
Page 20: Result View
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Figure 13. Home view 5.5. Result view To access the results from the Home view, tap the “View test results” button on the bottom of the slot pane (see Figure 14). This brings you to the Results view. Alternatively, result history is available by tapping the “Results” button at the top of the screen. - Page 21 Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 A different result view is be displayed depending on which test has been run (see Figure 15). The information displayed are: Sample ID Test type Date, time and user ...
- Page 22 Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Figure 17. Result View - Details The run history can be navigated by using the scrollbar. Tap the run in the list to view the results of the selected run (see Figure 15). Runs can also be filtered by tapping the “Edit filters”...
-
Page 23: System Setup (Admin User)
Report as PDF Report as print Log file. Mobidiag representative may request you to deliver log files for troubleshooting failed runs (see Chapter 10). Figure 19. Result View – Exporting options As a default, the exported files (csv, PDF or logs) are saved to USB drive, if one is plugged in to the Touchscreen Computer. - Page 24 Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 5.6.1. Instrument Management Instrument Management view allows you to enable/disable Novodiag® Instruments. Tap the “ENABLED” or “DISABLED” buttons to enable/disable a Novodiag® Instrument. Disabled instruments are indicated by the X- symbol (see Table 6 in Chapter 5). Figure 20.
- Page 25 Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Figure 21. System Setup view - User Management tab 5.6.3. Settings In the Settings tab, you may adjust the automatic logout time, the export location for all export files and enable an automatic printing of the results.
- Page 26 An automatic printing of finished results can be taken in use by tapping “ENABLED”. If a default printer is set, the result is sent to the printer in PDF format. Contact Mobidiag representative for setting up the default printer. 5.6.4. Slot Management You can enable and disable slots by tapping the “Slot Management”...
- Page 27 5.6.5. Tests Management New tests are installed by Mobidiag representative at the same time the user training is performed. Once installed, test plugins can be enabled and disabled from the Test Management view by selecting the test and tapping the “ADD TEST”...
-
Page 28: Novodiag® Software Upgrades And Plugins
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Figure 27. System Setup view - Test Management tab 5.7. Novodiag® Software upgrades and plugins The Novodiag® Software and the Novodiag® Instrument firmware are upgraded by Mobidiag representative. Operating instructions 6.1. General precautions WARNING! Before running Novodiag®... -
Page 29: Starting A Run
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 After protective cover removal, check the integrity of the white membrane. Do not remove or damage the membrane. Check for cartridge leak or sample spills on the cartridge. In the case the cap is damaged or possibly contaminated, always use provided replacement caps in the ... - Page 30 Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Figure 29. Home view - Scanning cartridge Scan the sample identification barcode (see Figure 30) or type the identification manually. The sample identification can be entered manually by tapping “abc” button, typing the code and tapping “Enter”. NOTE The Novodiag®...
- Page 31 Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Figure 31. Home view - Inserting cartridge The Novodiag® Software selects the slot to run and opens it. Ensure the cartridge is closed properly with a cap. Insert the cartridge in the tray of the slot of the Novodiag® Instrument and tap “CLOSE DOOR” on the dialog window.
- Page 32 Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Figure 32. Home view - Start Run The run starts and the remaining time is indicated both using text and with a circular progress symbol (see Figure 33). NOTE The estimation of the remaining time is based on the run history for a given test. In case the type of test is run for the first time, the remaining time displayed may be inaccurate.
-
Page 33: Shutting Down The Novodiag® System
Instructions for Use for more information). A negative control is used after installing the instrument or after maintenance carried out by a Mobidiag representative, to ensure the proper working of the instrument. It is also recommended for use at regular intervals, according to laboratory guidelines. A negative control can also be used in case of suspected nucleic acid or sample contamination in the working area or equipment used. -
Page 34: Decontamination And Disposal
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 possibility of a defective cartridge or instrument failure must be considered. In this case, please contact Mobidiag representative. Decontamination and disposal 8.1. Decontamination and cleaning procedures WARNING! The decontamination and cleaning procedures are intended to restrain a possible spread of contaminants which may result from sample spillage or a cartridge leakage. -
Page 35: Novodiag® Instrument Or Slot Disabling
Decontamination procedure prior to Novodiag® Instrument maintenance WARNING! Before a Novodiag® Instrument is to be replaced or removed by a Mobidiag representative, its decontamination according to Chapter 8.1 must be applied, to prevent any risk of infection. CAUTION Mobidiag representative will take care of the Novodiag® Instrument replacement or decommissioning. -
Page 36: Disposal
Do not replace any cable by cables that are not supplied by Mobidiag. • Mobidiag intends to provide adapted power cables for different countries. If suitable power cable is not available, do not use an adapter without an approval from Mobidiag. -
Page 37: Electromagnetic Compatibility
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Do not open or remove the Novodiag® Instrument covers. Do not use cable extenders to increase cable length but only the power strip delivered with the instrument(s). Disconnect the Novodiag® Instrument from the power supply before cleaning. ... -
Page 38: Reporting Product Anomalies
9.5. Vigilance information In the unlikely case of a suspected hazardous situation being caused or affected by the use of Mobidiag products, please contact Mobidiag representatives directly and without delay. See contact information in the Important Information section. Additionally, local competent authorities have standard reporting forms available for reporting possible adverse effects of IVD products, and users should follow any local regulations or guidance provided by the local authorities. -
Page 39: Novodiag® Software Troubleshooting
Retry. If the problem persists, contact Mobidiag representative. No protocol found for cartridge Ensure that the related test has been installed. If not, contact Mobidiag representative. Retry to read the barcode. If the barcode seems damaged, enter the barcode manually. If the problem persists, contact Mobidiag representative. -
Page 40: Touchscreen Computer Troubleshooting
Novodiag® Software is restarted. Collecting log files for Mobidiag Support When a failure has occurred, insert a USB drive in the Touchscreen Computer, browse the run that has failed and tap “Export as…”... -
Page 41: Technical Data
Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 10.5.2. Reset default settings If the Barcode Scanner does not operate properly, default settings need to be reset by scanning the following code: Figure 35. Code to reset default settings After the reset of default settings, the standing mode need to be reset again (see Chapter 10.5.1). 11. - Page 42 Novodiag® System User Manual (NVD-SYST-UM) V4-0 – April 2020 Table 14. Touchscreen computer technical data Touchscreen Computer Motherboard Processor Intel Skylake U i5-6300U Chipset CPU Integrated System memory DDR3L 1600 MHz 8Gt Memory socket 1x SO-DIMM Graphic engine Intel(R) Graphics 520 (1 Gt) LCD touch panel LCD size 15.6"...
-
Page 43: Important Information
User Manual or Instructions for Use is done solely at the customer’s own risk. Mobidiag is not liable for any indirect or consequential damage. -
Page 44: Manual Version Information
Every effort has been made to ensure that the information provided in this document is accurate as of the date of publication. Updates can be occasionally made, in which case the publication date and the version number will be changed. To make sure you are using the latest version of the user manual, please contact your Mobidiag representative. - Page 45 Ordering information Novodiag® System Product catalogue number: NVD-INST-B NVD-DSW NVD-TSC-A NVD-BCS-A NVD-TSCA-A NVD-TSCP-A Go to our website www.mobidiag.com For more information: Mobidiag Ltd. Keilaranta 16A FI-02150 Espoo FINLAND www.mobidiag.com Tel: +358 10 5050 789 support@mobidiag.com...

Need help?
Do you have a question about the Novodiag NVD-INST-B and is the answer not in the manual?
Questions and answers