Summary of Contents for SONIVATE MEDICAL SonicEye Dual-Array Ultrasound System
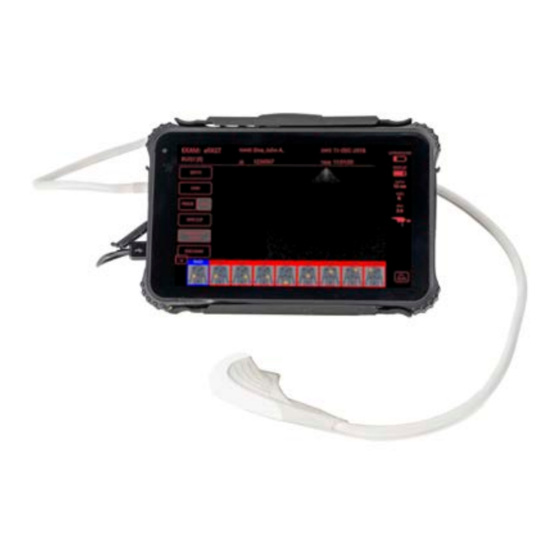
- Page 1 S O N I V A T E M E D I C A L , I N C . SonicEye® Dual-Array Ultrasound System Model# SDA-001 Part Number: 18210...
- Page 2 Regulatory Requirement This manual is a reference for the SonicEye® only. This manual is a reference for the SonicEye® software release 1.0. (SonicEye® is a Registered Trademark and exclusive property of Sonivate Medical, Inc.) Copyright ®Sonivate Medical, Inc. All Rights Reserved SonicEye Dual-Array Ultrasound System is protected under numerous Patents and Patents Pending.
- Page 3 SonicEye® User Manual Version 1.0 (continued) ii SonicEye – COUNTRY SPECIFIC APPROVALS AND ENVIRONMENTAL REQUIREMENTS Country Specific Approvals: United States of America Environmental Requirements: NOTE: Avoid exposing the unit to saline moisture. Requirement Temperature: Operational 0°C to 35°C (32°F to 95°F) Nonoperational -35°C to 65°C (-29°F to 149°F) Humidity 30% to 75% RH non-condensing...
-
Page 4: Table Of Contents
Table of Contents Chapter 1 - Introduction ......................t6 General Description ............................6 Ultrasound Principles of Operation ......................6 Safety ..................................6 Indication for Use ..............................6 Contraindication ..............................7 Conventions Used in this Manual ....................... 7 WARNINGS ................................8 Contact Information ............................8 Chapter 2 - Preparing the SonicEye for Use ................9 SonicEye Dual-Array Ultrasound System Package Contents .............9 System Description ............................9 First Time Use ..............................10... - Page 5 Table of Contents Chapter 6 - Safety ........................30 Introduction ..............................30 Owner Responsibility ............................ 30 Important Safety Considerations ......................30 Electromagnetic Compatibility (EMC) Safety ..................32 Medical Ultrasound Safety ...........................33 Device Labels..............................33 Chapter 7 - Appendix ............................35 Acoustic Output Reporting Tables ......................35 Measurement Uncertainties ........................35 Probe Temperature Data ..........................
-
Page 6: Chapter 1 - Introduction
Chapter 1 - Introduction CONTENTS: General Description Ultrasound Principles of Operation Safety Indications for Use Contraindications Conventions used in this Manual Warnings Contact Information GENERAL DESCRIPTION SonicEye® Dual-Array Ultrasound System employs an innovative and unique design that combines two transducers (high frequency linear and low frequency phased arrays) into a single, finger-mounted probe connected to an image processor. -
Page 7: Contraindication
CONVENTIONS USED IN THIS MANUAL Symbols used on the SonicEye® system and associated packaging are defined here for user reference. Manufactured for Sonivate Medical, Inc. By Valtronic Technologies (USA), Inc. Symbol... -
Page 8: Warnings
Keep the charger dry. Failure to observe this precaution may result in fire and electric shock • Keep this unit out of reach of children. Manufactured for Sonivate Medical, Inc. By Valtronic Technologies (USA), Inc. CONTACT INFORMATION 29200 Fountain Parkway... -
Page 9: Chapter 2 - Preparing The Soniceye For Use
6. One USB cable; one end is USB-A (larger) and one end is USB-C (smaller) Sonivate Medical, Inc. 4640 SW Macadam Avenue Suite 200 Manufactured for Sonivate Medical, Inc. NOTE: SonicEye Dual-Array Ultrasound System has a Portland, OR 97239 USA By Valtronic Technologies (USA), Inc. -
Page 10: Cradle Assembly
REGISTRATION Sonivate Medical, Inc. 4640 SW Macadam Avenue Suite 200 Manufactured for Sonivate Medical, Inc. Prior to charging the tablet and Image Processor go to www.Sonivate.com and register the product. Portland, OR 97239 USA By Valtronic Technologies (USA), Inc. 29200 Fountain Parkway... -
Page 11: Soniceye Activation
Chapter 2 - Preparing the SonicEye for Use (continued) Figure 2.1c Figure 2.1b Tablet AC Adapter: Model AW018WR-0500300UH; Input: 100-240V, 50/60Hz, 0.5A; Output: 5V, 3A. To achieve maximum charging capacity with your SonicEye Tablet and Image Processor batteries, you should allow the battery to be fully charged and then fully discharged at least three times. The unit can be used as normal during these cycles. -
Page 12: Graphical User Interface Overview
Chapter 2 - Preparing the SonicEye for Use (continued) CRADLE ASSEMBLY The SonicEye cradle holds the tablet and Image Processor in the shipping case (Figure 2.01). Detach the Image Processor by sliding it out of the cradle and insert the handles into the slots (Figure 2.02) on the tablet holder to form a kick stand (Figure 2.02). - Page 13 Chapter 2 - Preparing the SonicEye for Use (continued) The Settings section (Figure 2.4a) is where SonicEye can be customized to each user. Enter the Organization and User using a pop-up keyboard. The system will always operate in Landscape. The User may adjust left or right handed, in Operation as well as being to set the system on “Tactical”...
- Page 14 Chapter 2 - Preparing the SonicEye for Use (continued) The Scan: Manual section (Figure 2.7 shown in Tactical View) is for the more experienced user and it allows the freedom to switch back and forth between the phased array and the high frequency array with ONE TAP.
-
Page 15: Chapter 3 - Using Soniceye
Chapter 3 - Using SonicEye CONTENTS: Patient Data Entry Scanning Guided eFAST exam Manual Mode Recall and Transfer of Stored Data To Create a Report Deletion of Data Shut Down PATIENT DATA ENTRY: ONE TAP on the Patient ICON located on the HOME SCREEN. The Patient screen will appear. TOUCH the location of the Patient’s Last Name and a key board will appear. - Page 16 Chapter 3 - Using SonicEye (continued) CAUTION Coupling gels should not contain the following ingredients as they are known to cause probe damage: • Methanol, ethanol, isopropanol, or any other alcohol-based product • Mineral and Olive Oils • Iodine • Lotions including Aloe Vera, Lanoline, etc. Other Considerations: Like most high frequency computing devices, the electronic components of SonicEye will generate some heat while operating normally and as intended.
- Page 17 Chapter 3 - Using SonicEye (continued) Reference Low Frequency Marks Linear Array Stabilize with finger inserted in High Frequency elastomeric finger tip Linear Array Hold similar to traditional probes Figure 3.2 Pre-sets: SonicEye comes with already established pre-sets for frequency, depth and contrast (brightness). Only Depth and Contrast may be adjusted by the user within an eFAST exam.
-
Page 18: Scanning In Guided Efast Exam Mode
Chapter 3 - Using SonicEye (continued) The Dual-Array Probe has two probes built into one. It contains both a low frequency phased Gain Default Setting and Adjustment (Image Brightness/Contrast): Regardless of which mode the system is in, gain is pre-set for both arrays at “0”. These can then be adjusted accordingly by ONE TAP on the “Gain”... - Page 19 Chapter 3 - Using SonicEye (continued) Figure 3.4a Figure 3.4b Saving Images – Still images can only be saved after the image is frozen. To freeze an image while scanning your target structure, ONE TAP the “Freeze” button. While the image is frozen, the user can opt to “Unfreeze”...
-
Page 20: Scanning In Manual Mode
Chapter 3 - Using SonicEye (continued) When the user has completed the exam, ONE TAP the “End Exam” button. This will prompt a pop-up window that will allow the user to confirm the exam is complete, and provide options to continue the exam, save and exit, or abort the exam and exit, which will result in the exam not being saved. - Page 21 Chapter 3 - Using SonicEye (continued) Saving CineLoops or “Clips” – While scanning, the user can ONE TAP the “Save Clip” tab. Once the “Save Clip” tab is selected, the machine will then record the following 3 seconds of scanning and automatically store the clip in the window in which you are scanning.
- Page 22 Chapter 3 - Using SonicEye (continued) Deletion of Saved Patient Data The SonicEye system will automatically delete saved exams after 30 days from the date of examination. It will also delete the last saved exam by date and time when 30 exams are saved. An individual exam may be deleted using the TRASH icon.
-
Page 23: Chapter 4 - Soniceye Gui/App
SonicEye GUI PRE-INSTALLED The SonicEye GUI (graphical user interface) App will be installed on the supplied tablet. Sonivate Medical, Inc. User and Reference Manual are pre-installed in a PDF format. Updates may be attained through the Sonivate Medical, Inc. website. -
Page 24: Chapter 5 - Soniceye Maintenance
Chapter 5 - SonicEye Maintenance CONTENTS: System Care and Maintenance Inspecting the SonicEye Image Processor and Dual-Array Probe Cleaning and Disinfection Warranty Disposal Troubleshooting SYSTEM CARE AND MAINTENANCE The SonicEye requires regular care and maintenance to function safely and properly. To ensure that SonicEye consistently operates at maximum efficiency we recommend that the following procedures be observed as part of the customer’s internal routine maintenance program. -
Page 25: Soniceye Limited Warranty
Chapter 5 - SonicEye Maintenance (continued) CAUTION Do not spray any liquid directly onto the Image Processor DISINFECTING THE DUAL-ARRAY PROBE Ensure all solids are removed. After cleaning, the probe and cable may be wiped accordingly with a recommended disinfectant. Although a necessary step in protecting patients and employees from disease transmission, liquid chemical germicides must also be selected to minimize potential damage to the transducer. -
Page 26: Troubleshooting
Chapter 5 - SonicEye Maintenance (continued) TROUBLESHOOTING: Problem Possible Cause Solution Image Processor has no power Battery not charged Charge battery with supplier adaptor or use with power via adaptor Battery defect or end of life Contact SONIVATE Service Defect AC adapter or charger Contact SONIVATE Service Temperature is outside the specified limits Ensure the ambient temperature... -
Page 27: Chapter 6 - Safety
It is advisable to maintain a list of authorized operators. Should the system fail to operate Sonivate Medical, Inc. correctly, or if the SonicEye does not respond to the commands described in this manual, the operator... - Page 28 Chapter 6 - Safety (continued) PATIENT SAFETY Patient Identification Always include proper identification with all patient data. Identification errors could result in an incorrect diagnosis. It is the user’s responsibility that all the patient information is deleted from the tablet if the tablet is no longer being used for ultrasound purposes or returned to the manufacturer. WARNING The concerns listed in this section can seriously affect the safety of the patient undergoing a diagnostic ultrasound examination.
-
Page 29: Electromagnetic Compatibility (Emc) Safety
Chapter 6 - Safety (continued) CONTENTS: Personnel and Equipment Safety DANGER The hazards listed below can seriously affect the safety of personnel and equipment during a diagnostic ultrasound examination. The internal circuits of the AC/DC adapter use high voltages, capable of causing serious injury or death by electrical shock. -
Page 30: Medical Ultrasound Safety
Phone: 301-498-4100 or 800-638-5352 Fax: 301-498-4450 Device Labels The following two labels (Figures 6.1a and 6.1b) are attached to each device. Manufactured for Sonivate Medical, Inc. Sonivate Medical, Inc. By Valtronic Technologies (USA), Inc. 4640 SW Macadam Avenue 29200 Fountain Parkway... - Page 31 The following are attached to each secondary shipping container (Figure 6.2a and 6.2b): SonicEye Dual-Array ® Lithium Ion Battery Enclosed Ultrasound System P/N: 18210 Do Not Transport if Damaged Manufactured for Sonivate Medical, Inc. By Valtronic Technologies (USA), Inc. 29200 Fountain Parkway Solon, OH 44139 40034_2 40035_2 Figure 6.2a Figure 6.2b The following label (Figure 6.3) is attached to the back of the Sinicvision Technologies, 8-inch...
-
Page 32: Chapter 7 - Appendix
Chapter 7 - Appendix CONTENTS: Acoustic Output Reporting Tables Probe Temperature Data Terms and Conditions Acoustic Output Reporting Tables These tables show the highest possible acoustic intensity for a given mode, obtainable only when the maximum combination of control settings is selected. Most settings result in a much lower output. It is important to note the following: •... -
Page 33: Probe Temperature Data
Chapter 6 - Safety (continued) 1. Acoustic Impedance: = ±0.8 % 2. Variance is introduced by the stated temperature range of the water bath. 3. 5. Derating: = ±0.6 % 4. The contributing factor is error in the mechanical positioning of the hydrophone. The estimate is worst case by assuming a high frequency probe.

Need help?
Do you have a question about the SonicEye Dual-Array Ultrasound System and is the answer not in the manual?
Questions and answers