Table of Contents
Advertisement
Quick Links
INSTRUCTION
MANUAL
Multi-Mode
STIMULATOR
SPECIFICATIONS
Therapy Output Channels:
Therapy Output Modes:
Waveform:
Carrier Frequency:
Interference Frequency (IF):
Net Interferential Frequency:
Output Voltage:
Output Current:
Pulse Width:
Power System:
Dimensions:
Weight:
Preset Therapy Protocols:
1
REF: MMS Patient Manual
2 Therapy Channels
True interferential and conventional muscle stimulation
Symmetrical biphasic square wave with zero net DC
component
4000 Hz
4001 Hz to 4150 Hz
22.5 volts peak / 0 - 45 volts peak-to-peak, adjustable in 1/10
volt increments
0 - 45 milliamps, adjustable
125 micro seconds
2 AA batteries OR optional AC adapter
3.2" W x 4.8" L x 1.1" Thick
(81mm W x 122mm L x 27mm Thick)
6.0 oz. (167 grams) including batteries
14 (10 Interferential Stimulation, 4 Muscle Stimulation)
04-08-005 rev. 03
© 2018 Elite Medical Supply of NY, LLC
Advertisement
Table of Contents

Summary of Contents for Elite Medical Multi-Mode Stimulator
- Page 1 (81mm W x 122mm L x 27mm Thick) Weight: 6.0 oz. (167 grams) including batteries Preset Therapy Protocols: 14 (10 Interferential Stimulation, 4 Muscle Stimulation) REF: MMS Patient Manual 04-08-005 rev. 03 © 2018 Elite Medical Supply of NY, LLC...
-
Page 2: Table Of Contents
PRECAUTIONARY NOTICES This device is ONLY to whom it was prescribed. Use by any other person is prohibited and could result in injury. CAUTION: Federal Law (USA) restricts this device to sale by, or on the order of, a practitioner licensed by the state in which he or she practices to use or order the use of the device. -
Page 3: Package Contents
“seen” by the targeted injury site. This phenomenon of subtracting two frequencies to create a third frequency is called “beating”. The Multi-Mode Stimulator uses a carrier frequency of 4000Hz (hertz or cycles per second). At this frequency the skin impedance or resistance to the stimulation is at discomfort for the patient. -
Page 4: Indications For Use
The Multi-Mode Stimulator is a high-quality, advanced technology medical device designed to be used as a combination interferential and muscle stimulator. The Multi-Mode Stimulator is battery powered or power can be supplied with an AC wall adapter. Current is generated and controlled by the Multi-Mode Stimulator circuitry using Texas Instruments microprocessor chips. -
Page 5: Contraindications
CONTRAINDICATIONS Cancer patients and anyone with a demand type cardiac pacemaker should not use the Multi-Mode Stimulator. WARNINGS 1. The long-term effects of chronic electrical stimulation are unknown. 2. Stimulation should not be applied over the carotid sinus nerves, particularly in 3. -
Page 6: Adverse Effects
11. Electronic monitoring equipment (such as ECG monitors and alarms) may not operate properly when interferential stimulation is in use. in the management of pain patients. 14. The Multi-Mode Stimulator should only be used with the patient lead wires provided with the device or original manufacturer replacement patient lead wires. -
Page 7: Additional Features
Auto Shut-Off - To control the maximum treatment duration received by a patient, an automatic shut-off feature is incorporated into the Multi-Mode Stimulator. There are 20 and 30 minute time limits (depending on the selected preset) for each partic- ular therapy duration. After the session timer reaches zero, the output amplitude of minutes in an idle state, the device automatically shuts off to conserve battery power and prevent inadvertent operation. -
Page 8: Pre-Set Therapy Protocols
PRE-SET THERAPY PROTOCOLS Pre-set therapy protocols do not have variable adjustment parameters other than treatment amplitude (intensity). All functions are subject to treatment time. Dual Therapy - Output base frequency for set dwell time, then abrupt jump to high frequency for set dwell time, then repeat. After treatment time reaches ½ total treat- ment time, smooth ramp from 80 or 100Hz to 150Hz over set sweep time, then back. - Page 9 PRE-SET THERAPY PROTOCOLS (Continued) Treatment Protocol # Function Base Freq. High Freq. Sweep Time Timer Ramp 6 sec. Protocol 4 30 minutes 1 Hz 30 Hz 6 seconds “low” Ramp 12 sec. Protocol 5 30 minutes 80 Hz 150 Hz 12 seconds “high”...
- Page 10 PRE-SET THERAPY PROTOCOLS (Continued) Treatment Sweep or Protocol # Function Timer Base Freq. High Freq. Dwell Time Protocol 8 Abrupt shift 15 minutes 1 Hz 6 Hz 6 seconds Dual LOW Ramped shift 15 minutes 80 Hz 150 Hz 6 seconds Protocol 9 Abrupt shift 15 minutes...
- Page 11 PRE-SET THERAPY PROTOCOLS (Continued) Treatment Sweep or Protocol # Function Timer Base Freq. High Freq. Dwell Time Muscle 3 sec. ON / Protocol 11 20 minutes 50 Hz 50 Hz Simulation 3 sec. OFF Muscle 3 sec. ON / Protocol 12 20 minutes 50 Hz 50 Hz...
-
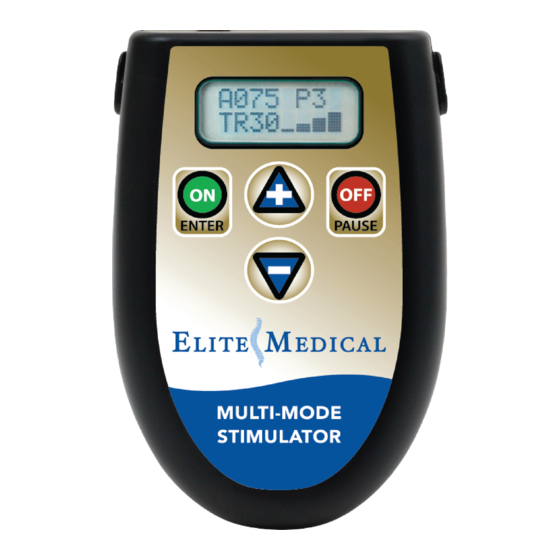
Page 12: Device Controls
ON / ENTER Button OFF / PAUSE button STOP! Do not use the Multi-Mode Stimulator until you have read the entire instruction manual, especially the warnings and precautions on pages 5-6. STANDARD OPERATION INSTRUCTIONS 1. -
Page 13: Quick Start Instructions
QUICK START INSTRUCTIONS Note to user: your device must be placed in therapy lockout mode by your health- care professional before you can utilize the quick start feature. Begin by following steps 1 and 2 in the Standard Operation Instructions section. To use the device in Quick Start mode simply turn the device on by pressing the ENTER/ON button. -
Page 14: Troubleshooting
TYPICAL DISPLAY SCREENS (Continued) This screen When the device SYSTEM *PAUSED* and the one is in the paused below will both state, the two FAILURE PRESS ON appear when screens to the device the left will be operating system detects a fault. displayed al- ternately. -
Page 15: Care And Maintenance
• Remove the batteries from the device during storage WARRANTY INFORMATION Elite Medical Supply of NY warrants the Multi-Mode Stimulator against any defects in materials and workmanship for a period of one year from the date the device is entered into service but no longer than 15 months from the date of sale. Warranty does not cover accessories such as wall adapter or patient lead wires. -
Page 16: Manufacturer Information
MANUFACTURER INFORMATION Manufactured by Elite Medical Supply of NY, LLC 1900 Ridge Road Suite #125 West Seneca, NY 14224 (866) 712-0881 For questions regarding proper use or to request more supplies, please contact Elite Medical Supply of NY at: (866) 712-0881...
Need help?
Do you have a question about the Multi-Mode Stimulator and is the answer not in the manual?
Questions and answers