Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Sedatelec Premio 30 laser duo
- Page 1 User manual Keep with your instrument English HME-PREMIO30-ENG ENGLISH 1/28...
- Page 2 Thank you for choosing the Premio 30 laser duo! Since it was formed, Sedatelec has based its work on studies carried complementary medicine, particularly those conducted by Dr P. Nogier, the father of auriculotherapy and auriculomedecine, to offer practitioners novel, highly reliable, high performance instruments.
-
Page 3: Table Of Contents
ABLE OF CONTENTS ......4 AFETY INSTRUCTIONS RESIDUAL RISKS AND PRECAUTIONS FOR USE & C ............... 7 NDICATIONS ONTRAINDICATIONS ............9 ESCRIPTION OF THE REMIO LASER DUO ............11 OW TO USE THE REMIO LASER DUO ................14 ECHNICAL SPECIFICATIONS &... -
Page 4: Safety Instructions
AFETY INSTRUCTIONS RESIDUAL RISKS AND PRECAUTIONS FOR USE The Premio 30 laser duo is an infrared laser which emits a laser beam at a rate of 905 nm. The Maximum Permitted Emissions (MPE) are 0.269 J/m the skin and 2.47*10 J/ m for the cornea. - Page 5 Not to store it at temperatures of under -20°C or over 50°C, Never to immerse it in a liquid. The Premio 30 laser duo contains a Li-Ion technology battery which must only be replaced by the staff instructed by Sedatelec.
- Page 6 For safety reasons, the instrument is provided with a laser key, which can restrict its use to authorised personnel. The Premio 30 laser duo requires technical safety checks at least every 24 months. These must be carried out by people who have knowledge, equipment and experience to carry out these checks (cf.
- Page 7 & C NDICATIONS ONTRAINDICATIONS Indications The Premio 30 laser duo is used for laser biostimulation (coherent light) or locally on painful pathological areas of the body. The indications are those recognized for acupuncture, Ear Acupuncture. Its use requires skilled staff who have sufficient knowledge of the indications, contraindications and medical risks of laser biostimulation, in a medical situation.
-
Page 8: Contraindications
Contraindications - Direct contact with mucosal membranes or broken skin. - Treatment of endocrine areas (particularly in children). - Treatment of the globe of the eye. - Treatment of abdomen in pregnant women. - Treatment of the chest region in a patient with a cardiac pacemaker. -
Page 9: Description Of The Premio
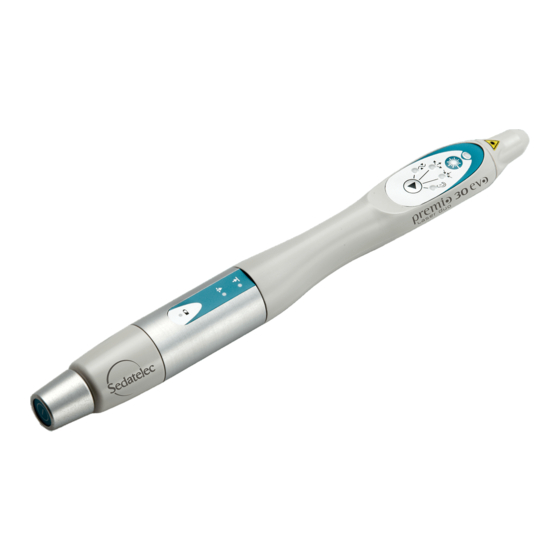
ESCRIPTION OF THE REMIO LASER DUO The Premio 30 laser duo is supplied in a bag containing: - A handpiece Premio 30 laser duo, - A specific laser key, - A test laser box, - A battery charger, - A pair of protective glasses with its manual,... - Page 10 Laser beam output: invisible laser radiation "Laser Danger" and "laser aperture" indicator Laser emission indicator Laser emission operating key Laser stimulation programme indicator: Harmonisation Acupuncture Dispersion Tonification Nogier frequencies scanning* Ear Acupuncture Type of laser stimulation selection key Superficial penetration indicator Deep penetration indicator Battery charge indicator ON/OFF key and selection key for penetration...
-
Page 11: How To Use The Premio
*: See the attached manufacturer's manual or on https://www.mascot.no/downloads/user-manuals/battery-chargers/ “chargers_li- ion”) In the event of a Battery fault: See chapter Troubleshooting. b. To check correct operation of the Premio 30 laser duo: Remove the laser key, Introduce the test laser plug into the instrument,... - Page 12 You should then contact your distributor or Sedatelec to have your instrument adjusted. Note: After using, disconnect the Premio 30 laser duo test laser in order that the battery does not discharge. Starting and operating information Insert the key into its socket on the back of the instrument.
- Page 13 Select the stimulation mode: press on the key to select desired programme: harmonisation, dispersion, tonification or Nogier frequency* scanning. In Acupuncture positions, to select the treatment depth press on the key to alternatively select laser penetration depth for superficial or deep points. Note: In the Ear Acupuncture position, depth is selected automatically.
-
Page 14: Technical Specifications
ECHNICAL SPECIFICATIONS Manufacturer Sedatelec Name of the device Premio 30 laser duo Type Infrared laser Emission features Type of laser diode InGaAs/GaAs Wavelength 905 nm Crest power 16.1 / 43.7 W (typical, not tested) Energy per pulse 1.125 / 3.6 µJ... -
Page 15: Frequencies & Programmes
We recommend routine disinfection of parts which may come into contact with patients. Your Premio 30 laser duo does not require specific maintenance and can be cleaned with a cloth soaked in soapy water, 70°C alcohol or with a cold disinfectant. -
Page 16: Troubleshooting - Recycling
ROUBLESHOOTING ECYCLING If the instrument does not function correctly, return it completely in its original bag to your retailer or to Sedatelec with your comments. When disposing of your Premio 30 laser duo, note that it contains electronic components and you should observe the current instructions for your country. -
Page 17: Appendix 1: Regulatory And Standard Related Information
1: R PPENDIX EGULATORY AND STANDARD RELATED INFORMATION Classification: The Premio 30 laser duo is a class IIa medical device according to directives 93 /42 EEC and 2007/47/EC. Applicable standards: - NF EN ISO 14971 :2013 - IEC 60825-1 :2014 - EN 60601-1: 2012 - NF EN1041 :2008. - Page 18 LABELS USED Positioning: on the device Serial number of the device Battery: Li-ion 3.6V 0.85Ah Charger: DC 4.2V 0.6A Positioning:: On food Product designation QR code + digits: UDI identifier * : Device reference : Name and address of manufacturer : Date of manufacture Positioning: On the box UDI identifier *...
- Page 19 Check crest power emitted by each pulse Technical safety check: Laser Test check Examination of the laser safety key We would thoroughly recommend that you perform these checks. Sedatelec is at your service to do these! HME-PREMIO30-ENG ENGLISH 19/28...
- Page 20 3: E PPENDIX LECTROMAGNETIC COMPATIBILITY Appropriate electromagnetic environment. The Premio 32D laser is suitable for use in all facilities including domestic and those directly connected to a low voltage public electrical supply network and domestic building supplies. The Premio 32D laser uses RF energy only for its internal operation.
- Page 21 Emissions and immunity test according to IEC 60601-1-2: Emissions tests Compliance Comments Emission of conducted CISPR 11 disturbance Class B, Group 1 Emission of irradiation CISPR 11 disturbance Class B, Group 1 Emission of harmonics Power less than Emission of Test not required as voltage/clashing instrument...
- Page 22 Manufacturer's recommendation and statement – electromagnetic immunity The Premio 30 laser Duo is designed for use in the electromagnetic environment specified below. The client or user of the Premio 30 laser Duo should ensure it is used in such an environment.
- Page 23 Electromagnetic Compliance with Level of Test of immunity environment IEC 60601 compliance recommendation Voltage drop, < 5% U < 5% U The quality of the power cut and (>95% drop of U (>95% drop of power supply must be power variations for 0.5 cycle ) for 0.5 cycle identical to a...
- Page 24 3 V/m 3 V/m closer than 30 cm (12 inches) from 80 MHz to 2.7 GHz Radiated RF any part of the Premio 30 laser duo, IEC 61000-4-3 including the cables, otherwise the Discrete 9, 27 or Discrete 9, 27...
- Page 25 4: I PPENDIX NSTRUMENT BOOKLET Type of instrument: Premio 30 laser duo Serial number: Manufacturer: Sedatelec Chemin des Mûriers F-69540 IRIGNY Distributor: Acquisition date: HME-PREMIO30-ENG ENGLISH 25/28...
- Page 26 VISUAL EXAMINATION Date Performed by Comments OPERATING CHECK Date Performed by Comments HME-PREMIO30-ENG ENGLISH 26/28...
- Page 27 ENERGY EMISSION CHECK Date Performed by Results Comments TECHNICAL SAFETY CHECK Date Performed by Comments HME-PREMIO30-ENG ENGLISH 27/28...
- Page 28 Premio 30 laser duo guarantees you the results of traditional practice. It frees you from all the restrictions and unpleasant aspects of punctures, inspires confidence to your patient and offers you comfortable working and entirely safe mastering of the therapeutic procedure.

Need help?
Do you have a question about the Premio 30 laser duo and is the answer not in the manual?
Questions and answers