Table of Contents
Advertisement
Quick Links
Biopsy System Instructions
Please read all information carefully.
Failure to properly follow the instructions may lead to serious surgical consequences.
IMPORTANT: This package insert is designed to provide instructions for use of the Mammotome elite Holster and Probe. It is
not a reference to surgical techniques.
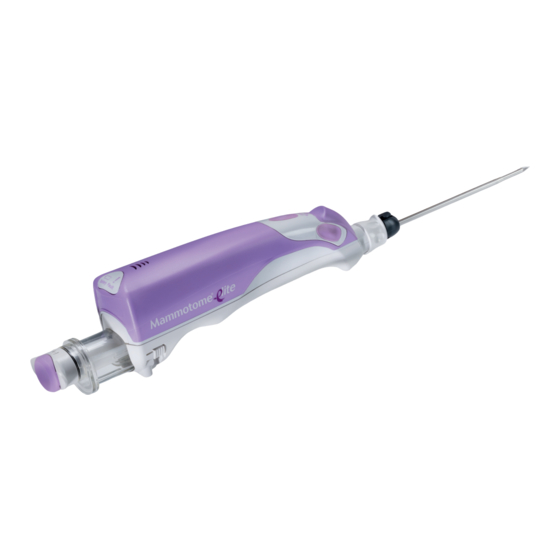
Mammotome elite is the only single-insertion, tetherless device with TruVac vacuum technology.
001212
www.mammotome.com/ifu
eIFU number:
Advertisement
Table of Contents

Subscribe to Our Youtube Channel
Summary of Contents for Devicor Mammotome elite
- Page 1 Please read all information carefully. Failure to properly follow the instructions may lead to serious surgical consequences. IMPORTANT: This package insert is designed to provide instructions for use of the Mammotome elite Holster and Probe. It is not a reference to surgical techniques.
-
Page 2: Indications For Use
None known. Device Descriptions Devicor’s Mammotome elite Biopsy System is composed of a Holster and a Probe. The System may also include the use of the optional accessory (the Introducer Stylet) to initiate the track within the breast tissue to the biopsy site (refer to Introducer Stylet IFU for details). - Page 3 (See Figure 3). IMPORTANT: The biopsy site identifiers may be used in conjunction with the Mammotome elite Biopsy System to radiographically mark the location of the biopsy procedure. DO NOT USE A MARKER NOT APPROVED FOR USE IN THE Mammotome elite BIOPSY SYSTEM.
- Page 4 Figure 2 Figure 3 Warnings • This instrument should only be used by physicians trained in percutaneous needle techniques for tissue collection. • As with any biopsy instrument, there may be a potential for infection. • Physicians should use sound judgement for use of this device with patients where increased risk or complications may be associated with core removal or biopsy.
- Page 5 Dispose of the Probe in an appropriate container. • The Probe is not designed for percutaneous skin puncture. • The Holster must only be used with Mammotome elite Biopsy Probes. • Dispose of all opened disposable instruments whether used or unused.
-
Page 6: Adverse Reactions
• Use of products manufactured or distributed by companies not authorized by Devicor Medical Products, Inc. may not be compatible with the Mammotome elite System and may lead to unanticipated results, EMC issues and possible injury to the user or patient. -
Page 7: Equipment Required
Illustration 2: Probe Illustration and Nomenclature Equipment Required • Appropriate imaging modality and accessories • Mammotome elite Biopsy Holster • Mammotome elite Biopsy Probe • Surgical gloves and drapes • Local anesthetic • Scalpel • Other equipment as necessary DIRECTIONS FOR USE... -
Page 8: First Time Use
• Holster: Follow Cleaning the Holster and Disinfecting the Holster procedures. • Probe: Dispose as it is no longer a sterile instrument. Verify compatibility of all minimally invasive instruments and accessories prior to using the instrument (Refer to Warnings and Precautions Sections). First Time Use Holster ships without a full battery charge. - Page 9 Illustration 3: CAUTION: To avoid pinching fingers while loading the Probe into the Holster, grasp the Holster on the sides above the logo and near the Probe Locking Tabs. 4. When the probe is inserted, the Holster will automatically turn on and begin an initialization sequence.
- Page 10 Illustration 4: The Essential Performance of the Mammotome elite Holster is the activation of the sampling cycle only when the active sample button is pressed. Procedures for Tissue Sampling Prepare the percutaneous site in accordance with standard surgical technique prior to insertion of the Probe.
- Page 11 6. Remove the protective sleeve from the Probe. • The Integrated Coaxial Cannula may be removed if further ease of insertion is desired. If it is removed, discard appropriately. It is recommended that the Integrated Coaxial Cannula not be reattached to the Probe to minimize the risk of injury.
- Page 12 • Push the active Biopsy Button only once to initiate the biopsy cycle. To interrupt the biopsy cycle, push the Biopsy Button again when the cutter is retracting from position 1 to 3 (See Illustration 6) or when the cutter is advancing from position 3 to 2.
- Page 13 a. Using standard surgical technique, insert a compatible marker through the Coaxial Cannula, using ultrasound image-guidance to confirm that the desired depth is reached. b. When the desired depth is reached - as confirmed by ultrasound image- guidance - deploy the marker. c.
- Page 14 CAUTION: Do not recap Probe tip. The cleaning agents and disinfectants specified for use in these instructions have been validated for use with the Mammotome elite biopsy system. The use of cleaning agents or disinfectants other than those specified in these instructions should be assessed for equivalency before using.
- Page 15 including tools and solutions, may influence the wear of a device or equipment. There are no limitations for time of storage of any disinfected device prior to use. Additionally, no support systems are required. Cleaning the Holster Less than 45 minutes after each use, manually clean the Holster thoroughly utilizing a pH neutral enzymatic detergent.
- Page 16 If the Holster is disinfected improperly, the warranty may be void. Do not transport the device in a container prior to disinfection. Disinfected products should be stored in dry, clean environment, protected from contamination, direct sunlight, pest and extreme temperatures and humidity. The Mammotome elite holster has an expected life of 500 reprocessing cycles.
- Page 17 Charging the Holster Completely charge the Holster prior to first time use. The Holster utilizes a rechargeable lithium-polymer battery. The battery will require approximately 200 minutes to fully charge. The battery must be adequately charged prior to using the Holster for the first time and prior to beginning each procedure.
-
Page 18: Troubleshooting
• During a biopsy procedure, the Battery Indicator Light will illuminate solid green. If the Battery Indicator Light flashes green, the Holster has sufficient charge to complete one procedure. If the Battery Indicator Light illuminates a continuous yellow, the Holster will not function and must be placed on the Charging Base immediately. - Page 19 3. The Holster is not charging when placed into the Charging Base: Check that the AC power cord is securely inserted into the Charging Base and a wall outlet. Check that there are no obstructions over the charging connections on the Charging Base or Holster and then insert the Holster into the Charging Base.
-
Page 20: System Specifications
EMC information provided in this installation guide. The Mammotome elite Biopsy System is intended for use in the electromagnetic environment specified below. The customer or user of the Mammotome elite Biopsy System should assure that is used in such an environment. - Page 21 Guidance and manufacturer’s declaration – electromagnetic emissions The Mammotome elite Biopsy System is intended for use in electromagnetic environments specified below. The customer or user of the Mammotome elite Biopsy System should assure that is used in such an environment.
- Page 22 Electrical Fast ± 2KV, 100 KHz PASS The Mammotome elite Biopsy Transient / Burst IEC System is suitable for use in all 61000-4-4 establishments other than domestic and those directly connected to the...
- Page 23 If the measured field strength in the location in which the Mammotome elite Biopsy System is used exceeds the applicable RF compliance level above, the Mammotome elite Biopsy System should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the Mammotome elite Biopsy System.
-
Page 24: Disposal And Recycling Information
140°F (60°C). How Supplied • The Mammotome elite Holster is supplied non-sterile. • The Mammotome elite Probes are supplied sterile for single patient use and sold separately. Discard after use. Calling For Service Call 1-877-926-2666 (U.S. calls), or +1-513-864-9000 (International calls—English speaking only) or contact your local representative. - Page 25 Devicor Medical Products, Inc. (2) repaired or altered outside Devicor Medical Products, Inc.’s factory in a way so as to, in Devicor Medical Products, Inc.’s judgment, affect its stability or reliability, (3) subjected to improper use, negligence...
- Page 26 Devicor Medical Products, Inc. neither assumes nor authorizes any other person to assume for it any other liability in connection with the sale or use of any of Devicor Medical Products, Inc. products. There are no warranties that extend beyond the terms hereof.
- Page 27 E-mail: qualidade@imexmedicalgroup.com.br Mammotome and Mammotome elite are registered trademarks of Devicor Medical Products, Inc. in the USA and optionally in other countries. TruVac is a trademark of Devicor Medical Products, Inc. in the USA and optionally in other countries. Devicor Medical Products, Inc.

Need help?
Do you have a question about the Mammotome elite and is the answer not in the manual?
Questions and answers