Subscribe to Our Youtube Channel
Summary of Contents for Bomedent Actor I pro
- Page 1 Ultrasonic Endo Activation Device User Manual Changzhou Bomedent Medical Technology Co., Ltd.
- Page 2 Thank you very much for choosing the Ultrasonic Endo Activation Device of Bomedent To give full play to the function of this device, and operate and maintain it correctly and safely, please read this User Manual carefully before using it, and...
-
Page 3: Table Of Contents
Contents 1. Product Introduction ......................1 1.1 Brief Introduction ......................1 1.2 Model ..........................1 1.3 Scope of Application ....................... 1 1.4 Contraindications ......................1 1.5 Precautions for Use......................1 2. Product Configuration ......................3 2.1 External Structure of Main Frame of Product ..............3 2.2 Main Accessories of Product ................... -
Page 4: Product Introduction
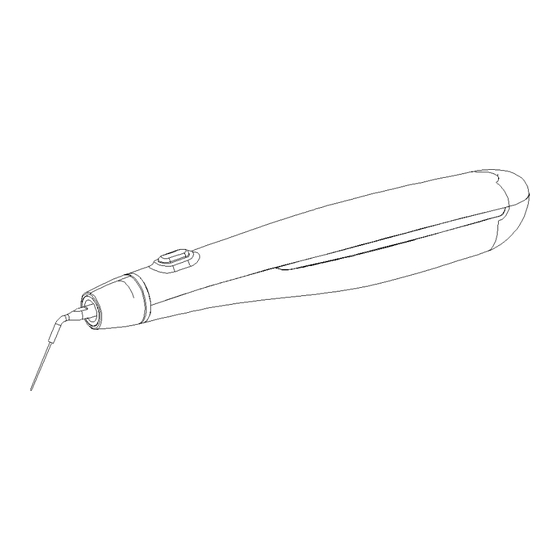
1. Product Introduction 1.1 Brief Introduction Ultrasonic Endo Activation Device (hereinafter referred to as “Actor I pro” or “device”) is an auxiliary device for dentists to perform root canal treatment. It is mainly used to effectively clean the root canal with the help of ultrasonic cavitation, so as to assist dentists to complete root canal treatment. - Page 5 This device may not work properly due to the following environmental factors: 1) Portable or movable radio frequency transmitters are present nearby. 2) Electromagnetic interference may cause abnormal operation of this device. As with all electronic devices, this device has electromagnetic interference and should not be used on patients with cardiac pacemaker.
-
Page 6: Product Configuration
2. Product Configuration 2.1 External Structure of Main Frame of Product 2.2 Main Accessories of Product Working tip Wrench Silicone case Power adapter Power cord Charging base Wireless foot switch (optional) -
Page 7: Accessories List
2.3 Accessories List Please see the packing list for the device configuration. 3. User Interface ① : switch button with built-in power indicator light ② : battery power indicator light and charging indicator light ③ : bluetooth button with built-in bluetooth indicator light ④... - Page 8 again to stop working. Power setting: press and hold the switch button for 1 second in the working state to switch between "high" power and "low" power Power indicator light: Under "high" power: the power indicator light is always green; ...
-
Page 9: Product Installation
charging base is always on in green; When the main frame is placed on the charging base to charge: the charging indicator light on the charging base is always on in blue. 4. Product Installation 4.1 Installation of Silicone Case Align the silicone case with the head and gently put it on. -
Page 10: Product Use
counterclockwise to loosen the working tip, and continue to rotate the wrench until the working tip comes out. Caution: The number of use of the working tip should not exceed 20 operations, with the number of 2 root canals per operation as the reference. Warning: ... - Page 11 always on in green; in the "low" power working state, the power indicator light flashes in green. Stop working: when pressing the switch button in the working state, the working tip will stop vibration, and the device will enter the standby state. The device will be powered off automatically after standby for 1 minute.
-
Page 12: Charging
Connect the USB cable to the charging base and turn on the power. Then put the main frame of Actor I pro into the charging base for charging. When the main frame is placed in the charging base for charging, the main frame will be powered off automatically. -
Page 13: Wireless Foot Control
Step on any button of the foot switch to open the power supply of the foot switch; Turn on the power of Actor I pro, press the bluetooth button ③, turn on the bluetooth, and the indicator light is always on in blue; Actor I pro will... - Page 14 After the connection is successful, the bluetooth indicator light flashes. After pressing and holding any button of the foot switch, Actor I pro starts to run; release the button to stop its operation; Caution: Only when the wireless foot switch is in standby state, the bluetooth can be turned on by pressing the bluetooth button of Actor I pro;...
-
Page 15: Troubleshooting
6. Troubleshooting Reference Fault Check items Fault analysis and handling method chapter or phenomenon section The battery power is low. Charge it in time. The device does not work after The time to press the switch starting up Please press and hold the switch button button is too short The wrong power adapter is Please use the original power adapter... -
Page 16: 7. Cleaning, Disinfection And Sterilization
Please do not use liquid or spray cleaner directly on the main frame. Please do not disinfect the main frame by heating; Parts of the Actor I pro that can be sterilized include the working tip, wrench, and silicone case The work tip, wrench and silicone case have biocompatibility (in line with standard EN ISO 10993-1). - Page 17 Wet the soft clean cloth in the cleaner, and then use it to wipe the surface of the working tip, wrench and silicone case thoroughly 5 times. Replace it with a new one after each wipe. If there Wipe are still visible contaminants, wipe repeatedly until no contaminants are visible to naked eyes. Scrub the working tip, wrench and silicone case thoroughly for 3 minutes with an instrument Scrub brush with cleaner.
-
Page 18: Storage, Maintenance And Transportation
8. Storage, Maintenance and Transportation 1. Storage This product should be handled with care, kept away from earthquake source, and stored in a dry and ventilated place. b. This product should not be placed together with toxic, corrosive, flammable and explosive items. The relative humidity of the storage environment should be 10%~80%, the atmospheric pressure should be 500-1060hPa, and the temperature should be -10℃~ +50℃. -
Page 19: Technical Parameters
9. Technical Parameters Manufacturer Changzhou Bomedent Medical Technology Co., Ltd. Product name Ultrasonic Endo Activation Device Actor Ⅰ pro Model Main frame: 169*26*28mm Dimensions Charging base: 82*64*73 mm Weight 700g Power supply mode Lithium battery, DC 3.7V, 1600mAh Input: DC5V/1A Charging base Output: DC 5V, 0.5A max... -
Page 20: Symbol Description
10. Symbol Description Warning: please read this manual Refer to the user manual before use Type B application device Class II device The product complies with the directive on WEEE. This device Serial number must be disposed of as municipal solid waste when abandoned Manufacturer Date of manufacture... -
Page 21: Emc Statement
13. EMC Statement Special precautions regarding electromagnetic compatibility (EMC) should be taken for this Ultrasonic Endo Activation Device, and it must be installed and used in accordance with the EMC information specified in this manual. Portable and movable RF communication equipment may affect this device. With the exception of cables (transducers) sold as spare parts for internal components, the use of accessories and cables (transducers) other than those specified may result in an increase in emission or a decrease in immunity of this device or... - Page 22 Guidance and manufacturer’s declaration – electromagnetic emissions The device is intended for use in the electromagnetic environment specified below. The customer or the user of the device should assure that is used in such an environment. IEC60601 test Immunity test Compliance Level Electromagnetic environment - guide level...
- Page 23 Guidance and manufacturer’s declaration – electromagnetic emissions The device is intended for use in the electromagnetic environment specified below. The customer or the user of the device should assure that is used in such an environment. level EN Compliance Immunity test Electromagnetic environment - guide 60601-1-2 Level...
- Page 24 Recommended separation distances between portable and mobile RF communications equipment and the device The device is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the device can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF according to the maximum output power of the communications equipment.
- Page 28 After-sales service company: Changzhou Bomedent Medical Technology Co., Ltd. Tel: 0519-88991980 ChangZhou BoMedent Medical Technology Co.,Ltd. NO.9 Changyang road, West Taihu Science & Technology Industrial Park, Changzhou City, jiangsu, China Caretechion GmbH Niederrheinstr 71, 40474 Duesseldorf, Germany Document Number: RD-RCW-030...



Need help?
Do you have a question about the Actor I pro and is the answer not in the manual?
Questions and answers