Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Neurotech kneehab
- Page 1 Instruction manual Caution: In the United States of America federal law restricts the device to sale or use by, or on the order of a physician or other practitioner licensed by the laws of the state in which he/ she practices. Advanced Therapy for Knee Injuries...
- Page 2 Kneehab XP Control Unit, Kneehab XP garment, a set of adhesive electrodes, battery charger and a user manual. The adhesive electrodes need to be replaced periodically and are readily available by contacting your local neurotech supplier. ®...
-
Page 3: Table Of Contents
TABLE OF CONTENTS Safety Information ___________________________________________________ Contra-Indications ___________________________________________________ Warnings ____________________________________________________________ Precautions __________________________________________________________ Frequently Asked Questions __________________________________________ Getting to Know Kneehab XP ________________________________________ Kneehab XP Pack Contents ___________________________________________ Description of Controls _________________________________________________ Description of Unit Display ______________________________________________ Description of Garment ________________________________________________... -
Page 4: Safety Information
BMR Ltd., Parkmore Business Park West, Galway, Ireland. Copyright © 2007 by BMR Ltd., all rights reserved. neurotech is a registered trademark of BMR Ltd. Kneehab is a registered trademark of BMR Ltd. ®... -
Page 5: Contra-Indications
Contra-Indications • Powered muscle stimulators should not be used on patients with cardiac demand pacemakers / defibrillators. Warnings • The long-term effects of chronic electrical stimulation are unknown. • Stimulation should not be applied over the carotid sinus nerves, particularly in patients with a known sensitivity to the carotid sinus reflex. - Page 6 • For hygiene reasons, the garment and electrodes are for single-person use only. Important • There is a separate Kneehab XP garment for use with the right or left leg. Under no circumstances should the left garment be used on the right leg or vice versa. A letter printed on every garment (L or R) indicates which leg that garment is designed for i.e.
-
Page 7: Frequently Asked Questions
Electrical Muscle Stimulation (EMS) product designed specifically to treat quadriceps atrophy (thigh muscle weakness). Clinical research has shown EMS technology to be an efficacious treatment for quadriceps atrophy, which is a symptom of almost all knee injuries. Kneehab XP can be used both pre- and post-opera- tively to accelerate knee rehabilitation. - Page 8 Q: How should I care for my Kneehab XP A: See page 18 of the User Manual for full instructions on caring for your Kneehab unit Q: Can I move around while using Kneehab XP A: If you need to move around during a treatment session, press the On/Off (Pause) button briefly, to pause the treatment. To complete the treatment session, just press the On/Off (Pause) button a second time.
-
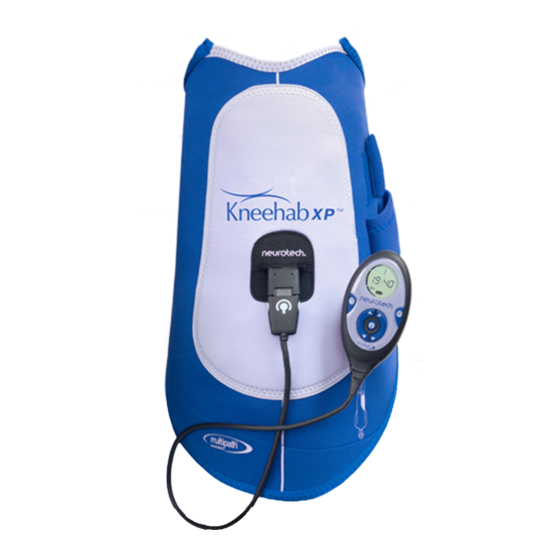
Page 9: Getting To Know Kneehab Xp
Getting to know Kneehab XP Kneehab XP is a portable, two-channel transcutaneous electrical muscle stimulator which operates using constant current pulses to stimulate the nerves in the quadriceps area of the body using neurotech ’s MULTIPATH™ technology. These pulses are ®... -
Page 10: Description Of Controls
Press and hold this button for 3 seconds before starting a treatment session to change the selected stimulation program. Note: Your prescribed Kneehab XP model may only have 1 stimulation programme. Press and hold this button for 3 seconds during a treatment session to activate/ deactivate the intensity button lock function. -
Page 11: Description Of Unit Display
Description of Unit Display The following icons appear on the Unit Display at various times throughout the treatment. Toning intensity from the left-hand side of the garment Toning intensity from the right-hand side of the garment Counts down the time left in the current session in minutes and seconds. Indicates that the session has been paused. -
Page 12: Treatment
(Fig. c). Please check now to ensure you have the correct garment for the leg to be treated. If the garment you have is not for the correct leg, please contact your prescribing clinician or the neurotech office. - Page 13 • Ensure the metal studs are completely covered by all of the electrodes. • Press the edges of each electrode firmly onto the garment to ensure good adhesion. • Replacement electrodes are available from your local neurotech supplier. See page 6.
-
Page 14: Step-By-Step Treatment Guide
Step-By-Step Treatment Guide Fig. a Step 1 - Garment Fitting Using mild soap and water, cleanse the area where you will be placing the garment. The electrodes do not adhere well to dirt, oil, creams or other cosmetics on the skin. Ensure the skin is dry. Remove the liners from the black side of each electrode (Fig. - Page 15 As you become more experienced using Kneehab, you will quickly find the levels which suit you best. • The treatment is delivered in contraction and relaxation cycles. See your Programme Information Card for more details.
- Page 16 Step 4 – Finishing Treatment & Storage Fig. a The treatment session is completed when the counter reaches zero. The unit will beep to indicate this. Switch the unit off by pressing the On/ Off button for 3 seconds. Should you forget to do this, the unit will switch off automatically after 20 seconds anyway.
-
Page 17: Problem Solving Guide
• Electrodes not fully in contact with firmly against the skin electrodes the skin • Contact your local neurotech supplier • Electrodes are worn for replacement electrodes • Lotions, pigmented areas or dry • Remove any lotions or oils by washing spots present on the skin •... -
Page 18: System Maintenance
Detach the Control Unit from the garment before cleaning. You should never let your Kneehab Control Unit get wet, but it may be cleaned using a soft cloth, lightly dampened in mild, soapy water. The garment is washable but you must first remove the Control Unit and electrodes. Replace the liners on the pat- terned side of each electrode until you are ready to use your Kneehab unit again. -
Page 19: Accessories
Access to the interior is not required for maintenance purposes. If your unit is damaged, you should not use it, but should return it to neurotech or your local distributor for replacement or repair. Repairs, service and modifications may not be carried out by anyone other than qualified service personnel authorised by neurotech. -
Page 20: Technical Specifications
Technical Information General Specifications: Product Type: 411 Intended use: Electrical Muscle Stimulator Waveform: Symmetrical bi-phasic square waveform when measured into a standard bodyload. Garment Material: Outer Fabric: 100% Nylon Inner Fabric: 70% Polychloroprene, 30% Polyurethane Binding: 82% Nylon, 18% Elastane Fastenings: 100% Nylon Environmental Specifications: Operating Range:... - Page 21 SN stands for “serial number”. human health. On the Kneehab Control Unit is the serial number specific to this unit. The letter preceding the number indicates the year of manufacture, where “L” denotes 2006, “M” denotes 2007 etc.
-
Page 22: Warranty
Warranty neurotech® offers a warranty of 1 year for the Kneehab garment and 2 years for the Kneehab control unit *. Should your Knee- hab develop a fault within the specified time, neurotech will replace or repair your Kneehab free of charge, provided the unit: •... - Page 24 Distributed by: Manufactured by: Bio-Medical Research Ltd. Parkmore Business Park West Galway Ireland www.neurotechgroup.com...





Need help?
Do you have a question about the kneehab and is the answer not in the manual?
Questions and answers