Summary of Contents for Sejoy BG-211b
- Page 1 BG-211b Blood Glucose Monitoring System Owner’s Booklet Advanced Diabetes Management System...
-
Page 2: Table Of Contents
Contents Introduction ························································ ······································· Important Safety information ···························· Important Health-Related information ························································· Limitations Getting to know your system ·································· ··················································· Control Solution ·········································· Meter (battery included) ························································ Test Strips ···················································· Lancing Device ···························································· Lancet Setting up your meter············································· ·········································· Setting the date and time ········································... - Page 3 ···················································· Caring for your system Troubleshooting ························································ Technical information ················································· ·························································· Specifications Disposing of the meter, test strips, lancets and batteries ····· Warranty ································································· Traceability ······························································· Performance Characteristics ········································ Symbol Index ···························································· FCC Information ························································...
- Page 4 Introduction Peel off the insulation film from battery compartment before first use. Thank you for choosing Sejoy BG-211b blood glucose monitoring Owner’s Booklet system. Before you start testing, carefully read this Intended Use It’s intended to be used for the quantitative measurement of glucose(sugar) in fresh capillary whole blood.
-
Page 5: Important Safety Information
Introduction Important Safety information Warning • During normal testing, any blood glucose meter or lancing device may come in contact with blood. All parts of the kit are considered biohazardous and can potentially transmit infectious diseases from blood borne pathogens, even after you have performed cleaning and disinfecting. -
Page 6: Important Health-Related Information
Introduction Important Health-Related information ·Patients undergoing oxygen therapy may receive inaccurate results. ·Some people with diabetes do not experience symptoms of low blood sugar (hypoglycemia). Others, such as children or people who are unconscious or have certain disabilities, may not be able to communicate their symptoms to caregivers. - Page 7 Introduction ·The system is designed for using with whole blood samples. Do not use serum or plasma samples. ·DO NOT test on neonatal samples (new born). ·Inaccurate test results may be obtained at high altitude more than about 3, 048 meters above sea level. ·Hematocrit range: 30% to 55%.
-
Page 8: Limitations
Introduction Limitations The interference listed below have been tested and shown no apparent influence on results at the normal or higher therapy levels. Acetaminophen 2. 0 mg/dL Levodopa No clear range of effective drug concentration Ascorbic acid 2. 0 mg/ dL Creatinine 1. -
Page 9: Getting To Know Your System
Getting to know your system INCLUDED WITH YOUR KIT a. Meter (battery included) b. Test Strips c. Lancing Device d. Sterile Lancet e. Control Solution Manufacturer of Lancing Device and Sterile Lancet Manufacturer of Lancing Device and Sterile LancetShandong Lianfa Medical Plastic Products Co.. -
Page 10: Meter (Battery Included)
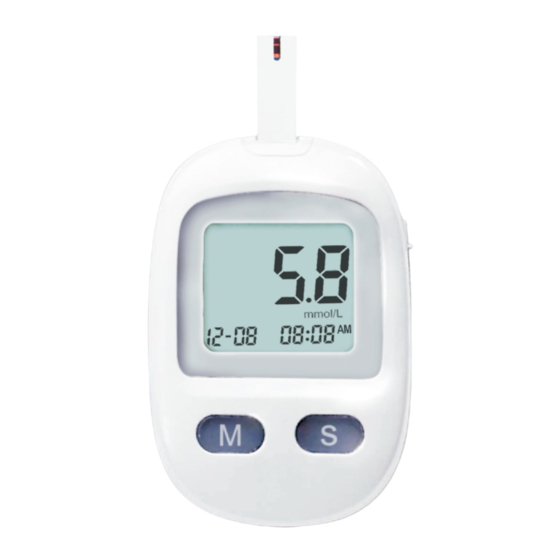
Getting to know your system a. Meter Strip Port Battery Cover Eject Button “S” Button “M”Button Battery Compartment Test Strip Reminder Apply Blood Sample Reminder Memory Temperature is out of range Low Battery Reminder Display Test Result Average Bluetooth mg/dL mmol/L Measuring Unit Time Alarm Clock Reminder... -
Page 11: Test Strips
Getting to know your system b. Test Strip Top Edge Contact Bars Confirmation Window Contact Bar: Insert it into strip port. Push it all the way until it will go no further. Top Edge: Apply blood sample here Confirmation Window: Sample checking area Important: the meter should only be used with BS-102 test strips. -
Page 12: Lancing Device
Getting to know your system c. Lancing Device Arrow Lancet Cap Trigger Button Sliding Barrel Adjustable Tip Lancing Device Cover Lancet Carrier Lancet Ejector d. Lancet Lancet Lancet Pin... -
Page 13: Setting Up Your Meter
Setting up your meter When use the meter for the first time, please set the parameters of the meter. With the meter off, long press button “S” to enter into setting mode. ① Set the month Press and release the button “M” to advance one month until the correct month appears . -
Page 14: Setting The Measuring Unit
Setting up your meter ④ Set the minute Press and release the button “M” to advance one minute until the correct minute appears. After the minute is set, press button “S”, the Time format figure will appear. ⑤ Set the time format The meter can display the time in either an AM/PM (12-hour) or a 24:00 (24-hour) format. -
Page 15: Setting The Test Reminder
Setting up your meter ⑦ Set the alarm clock The meter can set an alarm clock to remind you the time for testing. Press button “M” to check the alarm function. If the alarm is on, press button “S”, the time figure is flashing. -
Page 16: Bluetooth Connection
Testing your blood glucose Preparing the lancing device Warning • During normal testing, any blood glucose meter or lancing device may come in contact with blood. All parts of the kit are considered biohazardous and can potentially transmit infectious diseases from bloodborne pathogens, even after you have performed cleaning and disinfecting. - Page 17 Bluetooth connection Bluetooth requirements The Glucose Meter requires a bluetooth device with: . Bluetooth 4.0 or later . Android 6.0 or later . IOS 10.0 or later And works with: . iphone , iPod, iPad . Android Phones and Tablets Using for the first time 1.
-
Page 18: Testing Your Blood Glucose
Bluetooth connection Transfer your readings 1. As soon as your measurement is finished, open the app on your smart phone to transfer the reedings. Note: On the matched smart phone, Bluetooth must be enabled. Thermometer Blood pressure monitor After the measurement,the Glucose meter systemautomaticall analyzes Login... - Page 19 Testing your blood glucose Preparing the lancing device ① Twist off the lancing device cover. ① ② Insert a new lancet into the lancet carrier firmly. ③ Hold the lancet needle cover and gently twist it until it separates from the lancet. ④...
- Page 20 Testing your blood glucose Preparing the lancing device ⑥ Hold the lancing device cover in one hand. Using the other hand, slowly pull the sliding Barrel away from the lancing device cover. You will hear a click, indicating that the lancet carrier is locked into position.
-
Page 21: Blood Sampling
Testing your blood glucose Blood Sampling ① Wash your hands and the puncture site with an alcohol swab or soapy water. Rinse and dry thoroughly. ② Position the end of the adjustable comfort tip against the side-tip of the finger. Press the trigger button, and then lift the lancing device away from the finger after the puncture is complete. - Page 22 Testing your blood glucose Blood Sampling IMPORTANT: • Use only Test Strips BS-102. • Make sure your meter and test strips are about the same temperature before you test. • Testing must be done within the operating temperature range 10℃~40℃(50℉~104℉) For the most accurate results, try to test as close to room temperature 20℃...
-
Page 23: Testing
Testing your blood glucose Testing ① With meter off or in setting mode and memory mode, insert a test strip to enter into testing mode. Note: If you do not start the test within three minutes, the meter will turn off. To restart your meter, take out the unused test strip and reinsert it into the meter. - Page 24 Testing your blood glucose Testing ④ Apply the sample Gently touch the channel to the edge of the blood drop. Note: • Discard the first drop of blood. Do Not smear or scrape the drop of blood with the test strip. •...
- Page 25 Testing your blood glucose ⑧ Delete the memory If you don’t want to keep the test result, press the “S” button and “M” button to delete it at the same time. After memory cleared, the meter will display “dEL” for aprrox.3 seconds, and the nautomatically turn off. ⑨...
-
Page 26: Interpreting Unexpected Test Results
Testing your blood glucose Interpreting unexpected test results The meter can accurately measure blood glucose concentrations between 2.2 to 33.3 mmol/L (40 to 600 mg/dL). Expected Blood Glucose Level: Normal Blood Glucose Range Time Before breakfast 3.9-5.8 mmol/L(70-105mg/dL) Before lunch or supper 3.9-6.1 mmol/L(70-110mg/dL)... - Page 27 Testing your blood glucose Interpreting unexpected test results ② High glucose results If your test is above 33.3 mmol/L 600 mg/dL ), HI will appear on the display screen. This indicates severe hyperglycemia (high blood glucose). mmol/L • HI Reading with Symptoms If you feel symptoms such as fatigue, thirst, excess urination, or blurry vision, then follow your doctor’s recommendation to treat hyperglycemia.
-
Page 28: Comparing Your Meter Result To A Laboratory Result
The results of the Sejoy BG-211b meter are plasma equivalent. This method will help you and your healthcare professional compare your meter results w ith laboratory test results. The Sejoy BG-211b meter's test result and laboratory t e st results both are expressed in plasma-equivalent units. However, your blood glucose meter result may differ from your laboratory result due to normal variation. -
Page 29: Memory And Averages
Memory and averages Storing Blood glucose and Control Test results The meter automatically stores up to 360 blood glucose test results with the time and date of the test and any test markers. Results can be reviewed at any time. Test results are stored from the newest to the oldest, so set the time and date correctly in the meter. - Page 30 Memory and averages ③ Viewing7-, 14-, 28-day average Press and release “S” button to scroll forward to averages. The first memory display screen you see is your mmol/L 7-day average. This average includes all the readings from the last period days. n = indicates the number of results included in your average ④...
-
Page 31: Control Solution Testing
• Your test result does not match how you feel NOTE: • Only use the Sejoy CS-101 control solution. • Close the control solution bottle tightly after use • Write the date you open the control solution bottle on the bottle label. -
Page 32: Performing A Control Test
Control solution testing Performing a control test ① Insert a test strip to turn the meter on ② System check screen The backlight will be lit every time the meter is turned on. The screen will briefly display all content to confirm that the display is working properly. -
Page 33: Understanding Out-Of-Range Control Test Results
Control solution testing Performing a control test ⑤ Touch and hold the hanging drop of control solution where the narrow channel meets the TOP EDGE of the test strip. Make sure the confirmation window fills completely. Control solution should not be applied to the flat face of the test strip. - Page 34 • a problem with the meter. CAUTION: The control solution range printed on the test strip vial is for Sejoy Control Solution only. It is not a recommended range for your blood glucose level. CAUTION: If you continue to get control solution test results that fall outside the range printed on the test strip vial, Do Not use the meter, the test strips, or the control solution.
- Page 35 Control solution testing ⑦ Delete the memory If you don’t want to keep the test result, press the“S” and “M” button to delete it at the same time. After memory cleared, the meter will display “dEL”about 3 seconds, and then automatically turns off. ⑧...
-
Page 36: Maintenance
Maintenance Replacing the battery Your meter comes with one preinstalled, 3 volt, type 2032, lithium battery. The battery provides enough power for the meter to perform about 1000 tests. If your battery runs low, the battery symbol “ ” appears on every display screen until you change the battery. Important: When this symbol appears, you should replace the battery immediately. -
Page 37: Caring For Your System
Maintenance Caring for your system Avoid getting dirt, dust, blood, control solution, water, or any other liquid in the meter’s test strip port. Important: Never immerse the meter in water or any other liquid. This may cause inaccurate reading or meter malfunction. Storing your system Store your meter, test strips, control solution and other items in your carrying case after each use. - Page 38 Maintenance we recommend to disinfect periodically.When you assist others to make blood glucose testing, If you take the meter,please disinfect it or wear gloves to protect yourself. Cleaning your meter and lancing device To clean your meter and lancing device, wipe the outside with a soft cloth dampened with water and mild detergent.
-
Page 39: Troubleshooting
Troubleshooting Message Possible Cause What to do Remove the battery and The system check reinsert it after 30 seconds. may be failed If it still doesn’t work, please contact the vendor. Retest with a new strip. The test strip may be used or damaged. - Page 40 Troubleshooting Battery power is low. Replace the battery soon Place the system in The meter is out of appropriate operating operating temperature environment for 30 range. minutes before retesting.
- Page 41 Troubleshooting Meter does not enter the test mode after inserting a test strip. Probable Cause What to Do Replace the battery (and reset the date and The battery is dead. time,if necessary.) The battery is installed Check that the battery is installed correctly with the positive(+)sign facing upward, incorrectly or there is no toward you.
-
Page 42: Technical Information
Technical information Specifications Product description BG-211b Blood Glucose Monitoring System Assay method Glucose oxidase biosensor Measurement range 2.2-33.3 mmol/L ( 40~600 mg/dL Fresh capillary whole blood Sample Approximate 1 microlitre Sample size Response time 5 seconds Coding No coding Battery One 3.0V CR2032 lithium battery, replaceable... - Page 43 Technical information Specifications Weight Approximate 51g, battery not included 10℃~40℃(50℉~104℉) Operating environment 10 ~ 70% RH (non-condensing) -20℃~ 55℃ ( -4℉~131℉) Meter storage temperature 10 ~ 95% RH (non-condensing) 1℃~ 30℃ (33.8℉~86℉) Strip storage environment Up to 10,000 feet (3,048 meters) Altitude above sea level 30%~55 %...
-
Page 44: Disposing Of The Meter, Test Strips, Lancets And Batteries
Disposing of the meter, test strips, lancets and batteries Warning • Any product coming in contact with blood is considered contaminated (potentially infectious). • During normal testing, any blood glucose meter may come in contact with blood. • Lancing devices may also be considered sharps. Disposal of sharps is regulated by law in many jurisdictions. -
Page 45: Warranty
Warranty Limited 2-Year Warranty The meter is guaranteed for 2-year from the date of purchase. If the meter does not function properly due to defective components or poor workmanship, we will repair or replace it freely. This warranty does not cover damage due to improper handling in any way. -
Page 46: Traceability
Traceability The calibrator of the Sejoy Blood Glucose Monitoring System BG-211b is control solution. The traceability of the control solution is referenced to the EKF BIOSEN C line-Clinic glucose analyzer. The EKF is the reference method used to assess the accuracy with which glucose results are obtained using the system. -
Page 47: Performance Characteristics
Performance Characteristics The performance of the system has been evaluated both in laboratory and in clinical tests. Range: The display range of the meter is 2.2mmol/L to 33.3 mmol/L (40mg/dL to 600 mg/dL). "HI" and "Lo" messages indicate results outside of this range. •... - Page 48 Performance Characteristics Precision: Control Solutionav 2.41 mmol/L CV=5.2% Intermediate Precision Control Solutionav 7.51 mmol/L CV=3.3% Control Solutionav 20.73 mmol/L CV=2.6% Blood av 2.40 mmol/L CV=2.39% Blood av 5.01 mmol/L CV=2.39% Repeatability Blood av 7.51 mmol/L CV=2.41% Blood av 12.21 mmol/L CV=4.99% Blood av 20.74 mmol/L CV=5.00% To maximize your chances of an accurate comparison between meter and laboratory results, follow a few basic guidelines:...
-
Page 49: Symbol Index
Symbol Index Consult instructions for use In vitro diagnostic medical device Serial number Caution Batch code Manufacturer ∑ Contain sufficient for < n > tests Storage temperature limitation Sterilized using irradiation STERILE Do not reuse Use by... - Page 50 Symbol Index The Product confoems to the requirements of the EC Directive IVDD(98/97/EC) on in vitro diagnostic medical xxxx devices“xxxx” is the identification number of notify body Biological risks Keep dry Please dispose of the waste according to the local regulations Direct Current...
-
Page 51: Fcc Information
FCC INFORMATION Caution: Changes or modifications to this unit not expressly approved by the party responsible for compliance could void the user authority to operate the equipment. This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) this device may not cause harmful interference, and (2) this device must accept any interference received, including... - Page 52 Appendix 1. “FDA Public Health Notification: Use of Fingerstick Devices on More than One Person Poses Risk for Transmitting Bloodborne Pathogens: Initial Communication” (2010) http://wayback.archive-it.org/7993/20170111013014/http://www.fda.gov/ MedicalDevices/Safety/AlertsandNotices/ucm224025.htm 2. CDC Clinical Reminder:"Use of Fingerstick Devices on More than One Person Poses Risk for Transmitting Bloodborne Pathogens,(2010) https://www.cdc.gov/injectionsafety/Fingerstick-DevicesBGM.htmlfor Disinfection and Sterilization in Healthcare Facilities,Atlanta."...
- Page 53 Document No: DBG-1504-011 Version: Z Date of Issue: 20XX.XX Hangzhou Sejoy Electronics & Instruments Co., Ltd. Area C, Building 2, No.365, Wuzhou Road,Yuhang Economic Development Zone, Hangzhou City 311100 Zhejiang China Tel:(+86)571-81957767...













Need help?
Do you have a question about the BG-211b and is the answer not in the manual?
Questions and answers