Summary of Contents for Pac-Dent PROMATE CL
- Page 1 Instruction Manual (Please carefully read instruction manual before first use.) 670 Endeavor Circle, Brea, CA 92821 info@pac-dent.com (909) 839-0888 www.pac-dent.com #08IFUPMCL100 Rev.050421...
-
Page 2: Table Of Contents
Contents 1. Product Introduction 2. Installation 3. Function & Operation of Product 4. Operation Instruction 5. Troubleshooting 6. Cleaning, Disinfection, & Sterilization 7. Storage, Maintenance, & Transportation 8. Environmental Protection 9. After-Sales Service 10. Symbol Instruction 11. Statement 12. EMC-Declaration of Conformity 13. - Page 3 This equipment has been designed and developed in IEC 60601-1 compliance with Electric Safety standard IEC 60601-1 EN ISO 13485 enforced. It was designed and manufactured in accordance with an EN ISO 13485-certify quality assurance system.
-
Page 4: Product Introduction
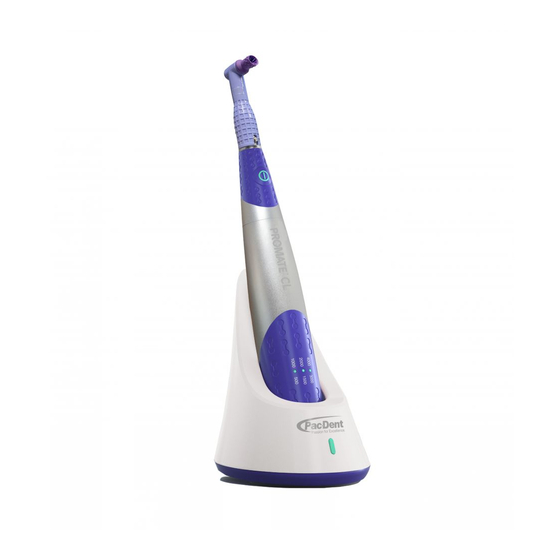
1. Product Introduction 1.1 Preface Thanks for purchasing the Pac-Dent ProMate™ CL Cordless Hygiene Handpiece. The ProMate™ CL medical device that you are about to install and use in your practice is a medical device designed for professional use. It comprises the chosen tool with which you will provide treatment within the context of your work. - Page 5 Do not perform repairs or make changes to the device without prior consent from Pac-Dent. • In the event of an abnormality, contact Pac-Dent. Do not call any other repair service, which may cause your device to be dangerous to you and your patients.
- Page 6 1.3 Operator population Operation of this medical device is limited only to certified, capable, and qualified dental professionals in their regular place of business. The operator must master and comply with the rules of dental practice in accordance with science and principles of medical hygiene, such as the cleaning, disinfection, and sterilization of medical devices.
- Page 7 This medical device may be used regardless of the characteristics of the patient, such as weight (except for children), age, height, sex, and nationality. Operation of this medical device is prohibited in the following patient population: implanted heart pacemaker patients (or other electrical equipment), patients who had been cautioned against the use of small electrical appliances (such as electric shavers, hair dryers, etc.), infants, pregnant or lactating women, patients with medical complications, allergic patients, patients with a clinical site not suited for...
- Page 8 1.7 Interactions • The device conforms to applicable electromagnetic compatibility standards. Operators must ensure that no electromagnetic interference causes an increased risk (presence of radio- frequency emissions, electronic devices, etc.). • Interference may occur when used on patients wearing a pacemaker. This system emits electromagnetic fields, which present potential risks.
- Page 9 1.9 Model and specification ProMate™ CL Cordless Hygiene Handpiece Please refer to the packing list for device configurations. Section 1.6 Principal of operation of the medical device. 1.10 Components • 1 Handpiece (motor) The handpiece houses the motor, gear box, power supply (lithium-ion battery) and three indicator lights, and is not sterilizable.
- Page 10 • 1 Inducting Charging Base Induction charge base allows the handpiece to be placed into the charger without hard connections. REF#: PMCL-104 • 1 AC Adapter. REF#: PMCL-105 • 1 Pack of 100 Disposable Plastic Barriers (placed over the handpiece and under the outer sheath for infection prevention).
- Page 11 1.11 Scope of application It is mainly used for teeth cleaning and polishing. The device must be operated in hospital and clinic settings by the qualified dentists. 1.12 Contraindications • Doctors with a pacemaker are prohibited from using this device. •...
- Page 12 • Long term use of equipment may result in overheating of motor handpiece, thus it should be left to cool for next use. If the motor handpiece is overheated frequently, please contact Pac-Dent. • Use the original charging base. •...
- Page 13 • Error in replacing detachable components can lead to unacceptable risks. Replace the detachable components according to the correct steps in the instructions. • Wireless charging will generate heat, and the surface temperature of the charging base and motor handpiece will rise. It is recommended that the time of contacting motor handpiece and charging base during wireless charging should not exceed 10 seconds.
- Page 14 Handpiece IPX3 Foot Control IPX1 Charging Base IPX0 Power Adapter IPX0 1.13.5 Degree of safety application in the presence of anesthetic mixture with air, oxygen, or nitrous oxide: Equipment cannot be used in the presence of a flammable anesthetic mixture with air, oxygen, or nitrous oxide.
- Page 15 1.15 Environment parameters • Environment temperature: +5 - +40℃ • Relative humidity: 30-75% • Atmospheric pressure: 70-106kPa...
-
Page 16: Installation
2. Installation 2.1 Basic accessories of product Disposable Prophy Angles Power Station REF# PMCL-104 Foot Control REF# PMCL-103 Power Adapter REF# PMCL-105 Outer Sheath REF# PMCL-102 Motor Component REF# PMCL-101... - Page 17 2.2 Instructions for Disposable Barrier 2.2.1 Before installing the disposable barrier, make sure that the motor handpiece is fully charged prior to operation. If it is of low battery level, please charge the device first. • Be sure to plug the AC adapter into the appropriate electrical outlet. •...
- Page 18 2.4 Instructions for Disposable Prophy Angle (DPA) 2.4.1 Install the disposable prophy angle (DPA) into the cordless handpiece. • Please use a disposable prophy angle (DPA) fitting this device. • Before installing the DPA, please ensure the motor handpiece has stopped. 2.4.2 Charge the foot pedal battery connecting it to the AC adapter cable for at least 2 hours.
-
Page 19: Function & Operation Of Product
3. Function and Operation 3.1 Button definition and settings Centralized Control Button Outer Sheath 3.1.1 Power on Press the Main Button to turn on the handpiece. 3.1.2 Power off Hold down the Main button to turn off the handpiece. 3.1.3 Handpiece speed setting With the handpiece turned on, press the Main button to adjust the speed mode. - Page 20 3.2 Function and operation of foot control 16 3.2.1 Activate the foot control Press the pedal for 3 seconds to activate the pedal. When the wireless connection indicator or power indicator of the pedal is on, it means that the pedal is activated successfully. 3.2.2 Foot control indicator The foot control has two indicator lights, the wireless connection indicator and the power indicator.
-
Page 21: Operation Instruction
4. Operation instruction 4.1 Power on Turn on the handpiece by short pressing the ON/OFF button. Turn the foot pedal on by stepping on it fully for 3 seconds (handpiece and foot pedal will automatically pair with Bluetooth connection). 4.2 Load polishing paste Adjust the handpiece to pedal mode or low speed mode, and then load the polishing paste to the Disposable Prophy Angle. - Page 22 4.5.2 Charging of wireless foot control Charge the wireless foot control directly with the original power adapter. • For safety reasons, the motor cannot be started when charging. • Please use the original power adapter for charging. Otherwise, the device may be damaged.
-
Page 23: Troubleshooting
5. Troubleshooting Failure Solutions Ensure that the outer sheath and the Disposable Prophy Angle are snapped together securely. Ensure that the Motor Component and the outer sheath are Disposable Prophy Angle is not rotating snapped together securely. Ensure the Disposable Prophy Angle is not damaged. Ensure the outer sheath is not damaged. -
Page 24: Cleaning, Disinfection & Sterilization
6. Cleaning, Disinfection & Sterilization 6.1 Foreword For hygiene and sanitary safety purposes, the outer sheath must be cleaned, disinfected, and sterilized before each use to prevent any contamination. This concerns the first use, as well as all subsequent uses. 6.2 General recommendations 6.2.1 Only use a disinfecting solution which is approved for its efficacy (VAH/DGHM-listing, CE marking, FDA and Health Canada approval) and in accordance with the DFU of the disinfecting solution... - Page 25 • Do not sterilize the motor component, power adapter and charging base. 6.3.1 Pre-Op processing Before each use, the motor component, power adapter and charging base must be cleaned and disinfected. The specific steps are as follows: • The motor component, power adapter and charging base cannot be cleaned and disinfected with automatic equipment.
- Page 26 6.3.1.2 Manual disinfection steps: Soak the dry, soft cloth with 75% alcohol. Wipe all surfaces of the motor component, power adapter, charging base and other components with a wet soft cloth for at least 3 minutes. Wipe the surface of the component with a dry, soft nap-free cloth. Note: •...
- Page 27 Wet the dry soft cloth with 75% alcohol, and then wipe all surfaces of the motor component, power adapter, charging base and other components for 3 minutes. Put the motor component, power adapter, charging base and other components back into the clean storage area.
- Page 28 use, thermal and chemical stresses will result in aging of the products. The maximum number of sterilizations for products is 250 times. 6.4.1 Initial processing 6.4.1.1 Processing principles It is only possible to carry out effective sterilization after the completion of effective cleaning and disinfection.
- Page 29 6.4.2 Preparation before cleaning Steps: Tools: tray, soft brush, clean and dry soft cloth. Remove the disposable prophy angle. Remove the outer sheath from the handpiece, and place them onto a clean tray. Use a clean soft brush to carefully brush the head and back cover of the outer sheath until the dirt on surface is not visible.
- Page 30 Notes: • The cleaning agent does not have to be pure water. It can be distilled water, deionized water or multi-enzyme. But please ensure that the selected cleaning agent is compatible with the product. • In washing stage, the water temperature should not exceed 45°C (113°F). Otherwise the protein will solidify and it will be difficult to remove.
- Page 31 Notes: • Before use, you must carefully read the operating instructions provided by the equipment manufacturer to familiarize yourself with the disinfection process and precautions. • With this equipment, cleaning, disinfection and drying will be carried out together. • Cleaning: (c1) The cleaning procedure should be suitable for the product to be treated. The flushing period should be sufficient (5-10 minutes).
- Page 32 6.4.5 Drying If your cleaning and disinfection process does not have an automatic drying function, dry it after cleaning and disinfection. Methods: Spread a clean white paper (white cloth) on the flat table, point the product against the white paper (white cloth), and then dry the product with filtered dry compressed air (maximum pressure 3 bar).
- Page 33 6.4.7 Packaging Install the disinfected and dried product and quickly package it in a medical sterilization bag (or special holder, sterile box). Notes: • The package used conforms to ISO11607. • It can withstand a high temperature of up to 138°C (280°F) and has sufficient steam permeability.
- Page 34 Notes: • Only products that have been effectively cleaned and disinfected are allowed to be sterilized. • Before using the sterilizer for sterilization, read the Instruction Manual provided by the equipment manufacturer and follow the instructions. • Do not use hot air sterilization and radiation sterilization as this may result in damage to the product.
- Page 35 6.4.10 Transportation Prevent excessive shock and vibration during transportation, and handle with care. It should not be mixed with dangerous goods during transportation. Avoid exposure to sun or rain or snow during transportation.
-
Page 36: Storage, Maintenance, & Transportation
7. Storage, Maintenance, & Transportation 7.1 Storage 7.1.1 This equipment should be stored in a room where the relative humidity is 10-93%, atmospheric pressure is 70kPa to106kPa, and the temperature is -20°C to +55°C (-4°F to 131°F). 7.1.2 Avoid storing in a too hot condition. High temperature will shorten the life of electronic components, damage battery, reshape or melt some plastic. -
Page 37: Environmental Protection
7.3 Transportation 7.3.1 Excessive impact and shake should be prevented in transportation. Lay it carefully and lightly and don’t invert it. 7.3.2 Don’t put it together with dangerous goods during transportation. 7.3.3 Avoid solarization and getting it wet in rain and snow during transportation. 8. -
Page 38: Symbol Instruction
10. Symbol Instruction Follow Instructions For Use Serial Number Date Of Manufacture Manufacturer Type B Applied Part Class II Equipment Used Indoor Only Recovery Handle With Care Keep Dry CE Marked Product Humidity Limitation Appliance Compliance WEEE Directive Protection Class IPX0 - IPX0 Classification Of Ingress Of Water For Charging Station – Not Protected Protection Class IPX1 - IPX0 Classification of Ingress Of Water For Foot Control... -
Page 39: Statement
Protection Class IPX3 - IPX3 Classification Of Ingress Of Water For Motor Component - Protected Against Falling Spray Temperature Limitation Atmospheric Pressure For Storage Authorized Representative In The EUROPEAN COMMUNITY 12. Statement All rights of modifying the product are reserved to the manufacturer without further notice. The pictures are only for reference. - Page 40 Technical Description Concerning Electromagnetic Emission Table 1: Declaration - electromagnetic emissions Guidance and manufacturer’s declaration - electromagnetic emissions The model ProMate™ CL is intended for use in the electromagnetic environment specified below. The customer or the user of the ProMate™ CL should ensure that it is used in such an environment. Emissions test Compliance Electromagnetic environment - guidance...
- Page 41 Technical Description Concerning Electromagnetic Immunity Table 2: Guidance & Declaration - electromagnetic immunity Guidance & Declaration — electromagnetic immunity The model ProMate™ CL is intended for use in the electromagnetic environment specified below. The customer or the user of the ProMate™ CL should ensure that it is used in such an environment. Environment.
- Page 42 Table 2: Guidance & Declaration - electromagnetic immunity - Continued IEC 60601 Environment. Compliance level Electromagnetic Environment - Guidance Test level Immunity test Voltage <5% UT (>95% dip in <5% UT (>95% dip Mains power quality should be dips, short UT.) for 0.5 cycle in UT.) for 0.5 cycle that of a typical commercial or hospital...
- Page 43 Table 3: Guidance & Declaration - electromagnetic immunity concerning Conducted RF & Radiated RF Guidance & Declaration - Electromagnetic immunity The model ProMate™ CL is intended for use in the electromagnetic environment specified below. The customer or the user of the ProMate™ CL should assure that it is used in such an environment. Immunity IEC 60601 Compliance...
- Page 44 Table 3: Guidance & Declaration - electromagnetic immunity concerning Conducted RF & Radiated RF - Continued. NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
- Page 45 Table 4: Recommended separation distances between portable and mobile RF communications equipment and the model ProMate™ CL Recommended separation distances between portable and mobile RF communications equipment and the model ProMate™ CL The model is intended for use in electromagnetic environment in which radiated RF disturbances is controlled.
- Page 46 Table 4: Recommended separation distances between portable and mobile RF communications equipment and the model ProMate™ CL - Continued For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) accordable to the transmitter manufacturer.
-
Page 47: Fcc Statement
14. FCC Statement This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This device may not cause harmful interference, and (2) this device must accept any interference received, including interference that may cause undesired operation. This device has been tested and found to comply with the limits for a WPC device, pursuant to Part 18 of the FCC Rules. - Page 48 Visit Pac-Dent's website for more information: www.pac-dent.com Manufactured for: Pac-Dent, Inc. 670 Endeavor Circle, Brea, CA 92821 (909) 839-0888 info@pac-dent.com www.pac-dent.com...

