Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Harvard Bioscience BTX AgilePulse ID
- Page 1 AgilePulse ™ In Vivo Gene Delivery System US E R ’S MA NU AL...
- Page 2 AgilePulse ™ ID In Vivo Gene Delivery System RESEARCH ONLY BTX Harvard Apparatus 84 October Hill Rd Holliston, MA 01746, USA phone 1-508-893-8999 • fax 1-800-429-5732 • www.btxonline.com BTX Warranty Harvard Apparatus warranties the AgilePulse ™ In Vivo Gene Delivery System for a period of two years from the date of purchase.
-
Page 3: Table Of Contents
Table of Contents SUBJECT PAGE 1 Introduction 2 Items of Particular Interest 3 AgilePulse™ Tutorial 3.1 Application 3.2 DNA Delivery Methods 3.3 DNA Delivery into Cells Using Electroporation 3.3.1 General Electroporation Discussion 8-10 3.3.2 AgilePulse™ Electroporation 11-12 3.3.3 Specific References 4 AgilePulse™... - Page 4 Table of Contents LIST OF FIGURES PAGE Electroporation Transmembrane Voltage Two Needles per Row Field Electrical Field Coverage Bleb Formation Waveform Generator AgilePulse Front Panel Login Screen System OK Screen Self Check Not Successful Screen IDA Electrode Handle IDA Tip Attaching IDA Tip to Handle User Login Screen Opening Screen...
- Page 5 Caution Notice This instrument contains a high voltage power supply adjustable to 1,000 volts. High voltage power supplies present a serious risk of personal injury if not used in accordance with design and/or use specifications, if used in applications on products for which they are not intended or designed, or if they are used by untrained or unqualified personnel.
-
Page 6: Introduction
Introduction The AgilePulse ™ IM system was developed to deliver small molecules using pulsed electric fields (electroporation). The system has been designed for research purposes. The system has three components: the AgilePulse Waveform Generator, the Intramuscular Array (IMA) electrodes (4 & 6) parallel needle arrays and the User Manual. Using other components may damage the system, will certainly provide degraded performance and invalidate the warranty. -
Page 7: Items Of Particular Interest
Items of Particular Interest The AgilePulse ™ ID DNA Vaccine Delivery System is a sophisticated system designed for delivery of DNA vaccines to the skin. The following are important: 1. The system shall only be used as described in this User Manual, the user is required to read the User Manual and undergo training before use. -
Page 8: Application
AgilePulse Tutorial ™ 3.1 Applications The AgilePulse ™ system is used to deliver DNA vaccines to study the effects and potential of this application for research purposes only. The AgilePulse ™ system has been shown to increase DNA delivery to cells resulting in 100-1000 times higher protein expression compared to hypodermic needle injection alone (Roos et al., 2006). -
Page 9: Agilepulse Tutorial
AgilePulse Tutorial ™ 3.3 DNA Delivery Into Cells Using Gene Electroporation Ions 3.3.1 General Electroporation Discussion Electroporation is the use of a transmembrane electric field pulse to induce microscopic pathways (pores) in a bio-membrane. Their presence Cell allows molecules, ions, and water to pass from one side of the membrane Membrane to the other. -
Page 10: General Electroporation Discussion
AgilePulse Tutorial ™ 3.3 DNA Delivery Into Cells Using Electroporation (cont’d) 3.3.1 General Electroporation Discussion (cont’d) Research in the late 1980s and early 1990s showed that certain experimental conditions and parameters of electrical pulses may be capable of causing many more molecules to move per unit time than simple diffusion. There is also good evidence (Sukharev et al., 1992) that DNA movement is in the opposite direction of the arrow in the sidebar. - Page 11 AgilePulse Tutorial ™ General References: Dimitrov, D.S., and Sowers, A.E., (1990) Membrane electroporation - fast molecular exchange by electroosmosis. Biochimica et Biophysica Acta 1022: 381-392. Sukharev SI, Klenchin VA, Serov SM, Chernomordik LV and Chizmadzhev YA, (1992) Electroporation, and electrophoretic DNA transfer into cells: The effect of DNA interaction with electropores, 1992, Biophys J. 63: 1320-1327.
-
Page 12: Agilepulse™ Electroporation
AgilePulse Tutorial ™ 3.3 DNA Delivery Into Cells Using The total treatment time for this waveform is 0.27 seconds (this waveform is currently tested in three clinical studies). When the Electroporation (cont’d) pulses are delivered in less than half a second there is only one muscle contraction and tolerability is highly improved. -
Page 13: Two Needles Per Row Field
AgilePulse Tutorial ™ 3.3 DNA Delivery Into Cells Using As the spacing between the rows increases the electric field rapidly falls off and cold spots form pores in the cells and vaccine Electroporation (cont’d) delivery does not occur. The AgilePulse ™... -
Page 14: Specific References
AgilePulse Tutorial ™ 3.3.3 Specific References There are ten specific references for this technique: Gehl, Julie, Thesis, Copenhagen, May 2002 Roos A-K, Moreno S, Leder C, Pavlenko M, King A, Pisa P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Molecular Therapy, 2006. 13; 320-327. Biragyn A, Schiavo R, Olkhanud P, Sumitomo K, King A, McCain M, Indig FE, Almanzar G, Baatar D. -
Page 15: Agilepulse™ System Components And Set-Up
AgilePulse ™ System Components & Set-Up 4.1 Introduction The AgilePulse ™ system provides the capability of delivering DNA vaccines to the dermis. The system is designed for easy set-up and operation. Specifications are presented in Appendices A and B, Tabs 7 and 8. The AgilePulse ™... -
Page 16: Front Panel
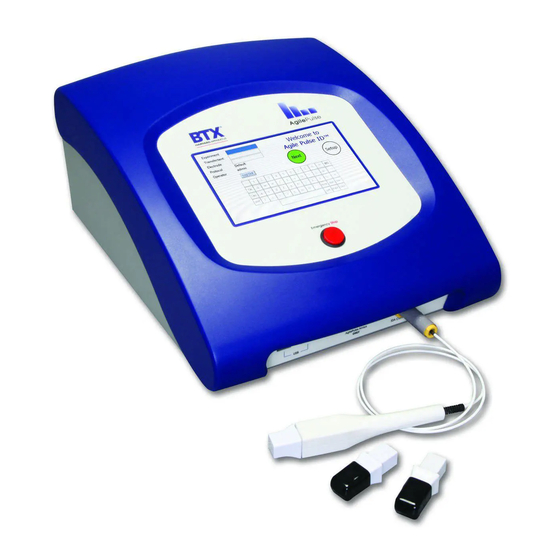
AgilePulse ™ System Components & Set-Up 4.2 The AgilePulse Waveform Generator (cont’d) 4.2.1 Front Panel The following can be found on the front panel: the Mains/Line power switch (illuminated when on), the Emergency Stop button switch, two USB ports, the ID Electrode Connector, and the User Touch Screen (Figure 4-2). -
Page 17: Setting Up The System
AgilePulse ™ System Components & Set-Up 4.4 Setting Up the System The system will be set up in the following sequence: 1. Unpack the contents of the shipping box. 2. Check for obvious signs of exterior damage. If damage is noted, contact BTX Customer Service before proceeding. -
Page 18: Touch Screen Cleaning
AgilePulse ™ System Components & Set-Up 4.4 Setting Up the System (cont’d) 4.4.2 Touch Screen Cleaning If the surface of the touch screen display needs to be cleaned, use a standard (non-ammonia) glass cleaner or mild detergent with warm water and a soft, lint free paper or cloth towel. Do not apply the cleaning solution directly to the screen, to avoid liquid running into other parts of the cabinet. -
Page 19: System Operation
System Operation This chapter describes the procedure to operate the Waveform Generator and to use the Intradermal Electrode. 5.1 Experiment Delivery The user operates the system via the front panel touch screen. When the power is turned on, the system boots up, and displays the opening screen. -
Page 20: Electrode Tip
System Operation 5.2 Intradermal Array (ID) Electrode 5.3 Waveform Generator (cont’d) 5.3.1 Login Screen – User Identification Input After power is turned on, a Login Screen is presented 5.2.2 ID Electrode Tip that requires the user to enter a user name and password The IDA tip is recommended for and is single use, disposable. -
Page 21: Identification Input
System Operation 5.3 Waveform Generator (cont’d) 5.3.2 Welcome Screen – Identification Input An opening screen (Figure 5-5) allows input of key experimental information after the initial system log in. Information is entered 5.3.1 Login Screen – User Identification Input by a virtual keypad. The purpose of this screen is for the USER (cont’d) (Researcher) to enter the Experiment ID, Transfectant and Electrode ID. -
Page 22: Delivery Screens Functions And Operations
System Operation 5.3 Waveform Generator (cont’d) 5.3.3 Experiment Delivery Screen Functions and Operation Figure 5-7: Delivery Screen - Load Estimation 3. If the LOAD Reading is within range (Less than 3500 ohms for skin) then the next step is to turn on the high voltage power supply by touching the READY button or depressing the foot pedal. -
Page 23: Opening Screen
System Operation 5.3 Waveform Generator (cont’d) 6. All run history log data are automatically stored in internal Flash Memory as well as a USB Key, if one is inserted. 5.3.3 Experiment Delivery Screen Functions and Operation (cont’d) Figure 5-10: Delivery Screen - Shutdown Figure 5-8: Delivery Screen - High Voltage Power Supply Charge 7. -
Page 24: Experiment Delivery Logs
System Operation 5.3 Waveform Generator (cont’d) 5.3.4 Experiment Delivery Logs All collected data from the therapeutic/vaccine delivery are automatically stored in the internal Flash Memory and an inserted USB key under a unique chronological directory structure. Included items are an XML file containing raw detailed system and runtime data, a text log with basic runtime data, and a comma-separated CSV file containing pulse monitor data. -
Page 25: Text Log
System Operation 5.3 Waveform Generator (cont’d) Field Description Pulse Pulse number 5.3.4 Experiment Delivery Logs (cont’d) VSet Voltage programmed to high voltage power supply VMon Voltage monitored during pulse 5.3.4.1 Text Log IMon Current monitored during pulse The text log contains the same information that appears on the RMon Equivalent load resistance calculated from voltage vaccine delivery process screen after completion. -
Page 26: Cvs Data
System Operation 5.3 Waveform Generator (cont’d) 5.3.4 Experiment Delivery Logs (cont’d) 5.3.4.2 CVS Data Detailed pulse information is exported from raw data and formatted to a standard comma separated file, primarily meant to be imported into Microsoft Excel ® for further user manipulation. As an example in Figure 5-12, a user chart was made to display the monitored amplitude samples of three 100 microsecond pulses run at 5 kHz. -
Page 27: Delivery Completion Return Codes
System Operation 5.3 Waveform Generator (cont’d) 5.3.5 Delivery Completion Return Codes Return codes are available following system shutdown describing how it took place. Return Code Description Successful completion (Normal Operation) Emergency Flash memory storage failure Waveform protocol data integrity verification error Waveform protocol mode not recognized Protocol voltage below minimum specification Protocol voltage above maximum specification... -
Page 28: Software Maintenance Mode
System Operation 5.3 Waveform Generator (cont’d) 5.3.6 Software Maintenance Mode The authorized system administrator is provided with the means to set system access privileges, add and delete pulse protocols, and access delivery log files. Non-administrator standard users can modify pulse protocols if given access by the administrator and retrieve previous therapeutic delivery logs. -
Page 29: Log Retrieval
System Operation 5.3 Waveform Generator (cont’d) 5.3.6 Software Maintenance Mode (cont’d) 5.3.6.2 Log Retrieval All previous therapeutic delivery logs can be retrieved and saved onto a USB Key or deleted from internal memory. The interface consists of a series of chronological sub-folders from which the user is allowed to double-tap on the touch screen to descend through the hierarchy. -
Page 30: Pulse Protocol
System Operation 5.3 Waveform Generator (cont’d) 5.3.6 Software Maintenance Mode (cont’d) 5.3.6.3 Pulse Protocol The pulse protocol to be delivered at run time is set by pressing the PROTOCOL button and adjusting parameters via the touch screen interface. Parameters for three pulse groups are modifiable by selecting a box and scrolling through the available values. -
Page 31: Customer Service
Customer Service 6.1 Limited Warranty The terms of the warranty are covered in the BTX warranty. 6.2 Customer Service If the user believes that there is a defect in the BTX product, the customer should contact BTX Technical Support at 800-272-2775 or email techsupport.btx@harvardapparatus.com. -
Page 32: Appendix
Appendix A: AgilePulse ™ ID Specifications Operation Delivery Electrode Vaccine Delivery Volume 2 blebs x 40 µl each IDA-4-6 Delivery 2 blebs x 45 µl each IDA-4-6 Touch Screen 2 blebs x 50 µl each IDA-6-6 Opening Screen for parameter entry Delivery Target Skin/dermis Experiment entry... - Page 33 Appendix A: AgilePulse ™ ID Specifications AgilePulse Waveform Generator Pulsing Front Panel Computer: Resistance Pulsing 4 µs at 5 volts every second Operating System Windows ® Mobile 6.0 Pulse Protocol Parameters Interface Touch screen Parameters in a Group: Line/Mains Switch with illumination Pulse Width 50 µs to 1 ms 50 to 1000 volts...
-
Page 34: Declaration Of Conformity
Declaration of Conformity Manufacturer: Harvard Apparatus, Inc. 84 October Hill Road Holliston, Massachusetts 01746-1388, U.S.A. Phone: (508) 893-8999 We herewith declare that the following product: BTX AgilePulse BTX AgilePulse BTX AgilePulse Product Name: ™ ™ ™ Model No.: Catalog # 47-04xx Catalog # 47-05xx Catalog # 47-02xx To which this declaration relates, is in conformity with the applicable EC Directives, harmonized...
Need help?
Do you have a question about the BTX AgilePulse ID and is the answer not in the manual?
Questions and answers