Advertisement
Quick Links
Advertisement

Summary of Contents for Eko Devices, Inc. Eko CORE
- Page 1 User Manual Model 2nd Generation © 2020 Eko Devices, Inc.
-
Page 2: Indications For Use
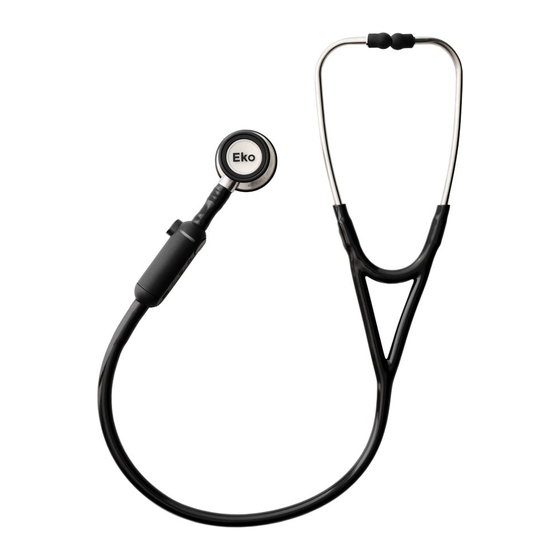
1. Indications for Use The Eko CORE is an electronic stethoscope that enables amplification, filtering, and transmission of auscultation sound data (heart, lungs, bowel, arteries, and veins), whereby a clinician at one location on network can listen to the auscultation sounds of a patient on site or at a different location on the network. - Page 3 2. Introduction The CORE is designed to support healthcare professionals in listening to sounds produced by the body, primarily lung, heart, and bowel sounds. CORE also enables regular users to record, store and share their body sounds with their physician. CORE includes a device that is attached to a stethoscope (CORE attachment) and an application, the Eko App.
-
Page 4: Equipment Symbols
4. Equipment Symbols Instructions for use European technical conformity 0537 European Authorized Representative Do not dispose with household waste Emits Radio Frequency signal Model number Humidity range 40°C Temperature range -30°C Wireless Bluetooth communication Manufacturer Manufacturing date Quantity IP22 IP22 indicates protection against access to hazardous parts with a finger, solid objects ≥... - Page 5 5. Cautions To reduce the risk of device interference, keep CORE at least 1 meter away from all RF emitters including Wifi routers and radios. Follow all cleaning and disinfecting instructions included in this manual. Establish and follow a cleaning and disinfecting schedule.
- Page 6 CORE contains a Bluetooth wireless data link. The maximum radio frequency field strength generated by the stethoscope is below three volts per meter, a level that is considered safe to use with other medical devices. However, audio, video, and other similar equipment may cause electromagnetic interference. If such devices are encountered and cause interference, immediately move CORE away from that device and/or turn the Bluetooth feature OFF.
-
Page 7: Emc Compliance
6. EMC Compliance FCC Intentional Radiator Certification Contains FCC ID: 2ANB3-E6 Contains IC: 23063-E6 47 CFR Part 15.105 required statement for Class B: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules. - Page 8 Canada regulatory statement(s): This device complies with Industry Canada license-exempt RSS standard(s). Operation is subject to the following two conditions: (1) This device may not cause interference; and (2) This device must accept any interference, including interference that may cause undesired operation of the device. Le présent appareil est conforme aux CNR d’Industrie Canada applicables aux appareils radio exempts de licence.
- Page 9 7. Contents and Operation CORE device includes (1) CORE attachment, (2) tubing adapters, and (1) micro USB cable and the Eko App. The compatible hardware and software platforms are listed below. Compatible Stethoscopes CORE is designed and tested to be compatible with the 3M™...
- Page 10 System Requirements The mobile app software can be used on iPhone 5S, iPhone 6/6 Plus, iPhone 6s/6s Plus, iPhone 7/ 7 Plus, iPhone 8/8 Plus, iPhone X, XS, XS Max, iPad* Mini 2/3/4, iPad Air/Air 2, iPad Pro, iPod Touch 6G, and iPad 5th and 6th generations with iOS 12.0 and higher.
- Page 11 8. Installation to Existing Stethoscopes This section is not required for pre-assembled digital stethoscopes Step One Grip chest piece with one hand and pull the tubing with force using the other hand to detach the chest piece from the tubing of the existing stethoscope.
- Page 12 9. CORE Use Charge Battery The battery in CORE will need to be charged; insert the included micro USB cable into the USB port on the device and plug the other end into a UL-certified USB wall charger. The LED will turn solid yellow, signifying that it is charging.
- Page 13 Setting up a PIN Create a secure 4-digit PIN by logging in to the mobile application. Navigate to the Menu screen by selecting the icon on the top left of the Mobile App home screen. Next, select Account Settings > Create Pin. Follow the instructions on the screen to create and save a 4 -digit PIN.
- Page 14 Figure 4a Figure 4b Headset alignment Before placing the eartips in your ears, hold the headset in front of you with the eartubes pointing away. Once the eartips are in your ears, they should point forward. Open the diaphragm When using a double-sided stethoscope (refer to Figure 3), you need to open (or index) the bell or diaphragm by rotating the chestpiece.
- Page 15 10. Cleaning Cleaning and Disinfecting Procedure The stethoscope and CORE should be disinfected between each use. Infection control guidelines from the Centers for Disease Control and Prevention (CDC) state that reusable medical equipment, such as stethoscopes, must undergo disinfection between patients. Standard stethoscope hygiene practices apply to the Eko device.
-
Page 16: Operating Conditions
11. Operating Conditions Environmental The operating temperature range of CORE is -30° to 40°C (-22° to 104°F), and 15% to 93% relative humidity. The storage and transport range is -40° to 55°C (-40° to 131° F), and 15% to 93% relative humidity. Acceptable pressure is 1 atm. Avoid exposure to extreme heat, cold, solvents and oils. - Page 17 13. CORE Modes and Corresponding LED States. CORE is on & seeking device (Blinking) CORE is on & connected CORE is recording (Blinking) CORE is low on battery (Blinking) CORE is off & charging CORE is fully charged 14. Eko App Download the Eko app, available on the App Store®...
- Page 18 14a. Eko App – Provider Workflow ➊ ➋ Sign up: Login: Create your Eko account by entering in name Enter in your login credentials and email address ➌ ➍ Turn on CORE Pair CORE...
- Page 19 14a. Eko App – Provider Workflow ➎ ➏ Start Recording: Save Recording: Place CORE on the patient’s chest; Press the Click save once your recording is complete blue button to start recording. ➐ Eko Settings Menu Adjust your settings by clicking on the ( ) top left home screen...
- Page 20 14b. Eko App – Patient Workflow ➊ ➋ Sign up Setup ➌ ➍ Turn on CORE Pair CORE...
- Page 21 14b. Eko App – Patient Workflow ➎ ➏ Start recording Recording in progress ➐ Recording complete...
-
Page 22: Electrical Safety
15. Electrical Safety Guidance and Manufacturer’s Declaration - Electromagnetic Emission The Eko Electronic Stethoscope System is intended for use in the electromagnetic environment specified below. The user of the Eko Electronic Stethoscope System should assure that it is used in such an environment. Applicable Compliance Electromagnetic... - Page 23 Guidance and Manufacturer’s Declaration - Electromagnetic Immunity The Eko Electronic Stethoscope System is intended for use in the electromagnetic environment specified below. The user of the Eko Electronic Stethoscope System should assure that it is used in such an environment. IEC 60601 Test Compliance Electromagnetic...
- Page 24 Guidance and Manufacturer’s Declaration - Electromagnetic Immunity The Eko Electronic Stethoscope System is intended for use in the electromagnetic environment specified below. The user of the Eko Electronic Stethoscope System should assure that it is used in such an environment. IEC 60601 Compliance Electromagnetic Environment-...
- Page 25 Recommended Separation Distances Between Portable and Mobile RF Communications Equipment and the Eko Electronic Stethoscope System The Eko Electronic Stethoscope System is intended for use in the electromagnetic environment in which radiated RF disturbances are controlled. The user of the Eko Electronic Stethoscope System can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Eko Electronic Stethoscope System as recommended...
- Page 26 16. Manufacturing and Regulatory Information Eko Devices, Inc. 1212 Broadway, Suite 100 Oakland, CA 94612 USA www.ekohealth.com 0537 Emergo Europe Prinsessegracht 20 2514 AP The Hague The Netherlands Australia Sponsor: Emergo Australia 201 Sussex Street Darling Park, Tower II, Level 20 Sydney NSW 2000, Australia LBL 071, Rev D - Issued Date: October 2020 ©...

Need help?
Do you have a question about the Eko CORE and is the answer not in the manual?
Questions and answers