Summary of Contents for Spirosure Fenom Pro
- Page 1 Asthma Monitor Instructions for Use IFU-3393 Rev C Instructions for Use, Fenom Pro,...
-
Page 2: Table Of Contents
Chapter 6: Quality Control Perform a QC Test QC User Status Create QC Users Chapter 7: Power Off Device Chapter 8: Practice Mode Chapter 9: Device Setup Initial Setup Configuration Settings Device Settings Time/Date Language IFU-3393 Rev C Instructions for Use, Fenom Pro... - Page 3 Exhaled NO Performance Linearity Precision Accuracy Limit of Detection Measurement Range Exhalation Parameters Chapter 13: Device Performance Accuracy Limit of Detection Chapter 14: Reference Symbol Explanation Chapter 14: Parts and Accessories Parts Accessories Bibliography IFU-3393 Rev C Instructions for Use, Fenom Pro...
- Page 4 Common office test used to assess how well a patient’s lungs work by measuring how much Spirometry air is inhaled, how much is exhaled, and how quickly it is exhaled. IFU-3393 Rev C Instructions for Use, Fenom Pro...
-
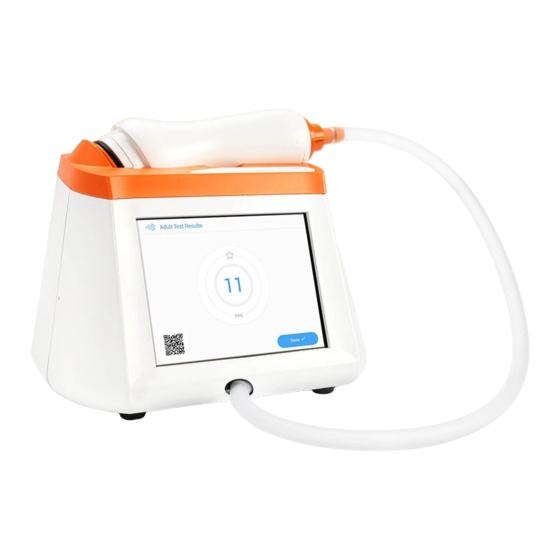
Page 5: Chapter 1: System Overview
Fenom Pro may not be used by children under the age of approximately 7 years, including infants, as measurement requires patient cooperation. Fenom Pro may not be used by children under the age of 7 years, or by patients who are unable to understand and execute the instructions given by healthcare providers, as measurement requires patient cooperation. - Page 6 Power Button – Hold for one second to power on/off. Single-Patient-Use Mouthpiece (accessory) Carrying Handle 24 V Power Connection IFU-3393 Rev C Instructions for Use, Fenom Pro...
- Page 7 Display Buttons There are several button icons that Fenom Pro utilizes to help you easily navigate through the menu screens. Table 2: Button and Indication Icons Buttons Icon Name Description Button used to open the Settings Menu. This menu allows for setting...
-
Page 8: Chapter 2: Safety And Warnings
Use only power supply unit provided. Keep the device out of water. Ensure no liquid is spilled or dripped on the device. DO NOT use the Fenom Pro device adjacent to or stacked with other equipment because it could result in improper operation. - Page 9 System Electromagnetic Immunity Test for RFID Readers. Fenom Pro is not compatible with magnetic resonance systems, and is labeled as MR Unsafe. The Fenom Pro should not be used in a room containing a magnetic resonance system or adjacent rooms to a magnetic resonance system.
-
Page 10: Chapter 3: Fenom Pro Quick Start Guide
Instruct the patient to keep the indicator over the star at the top of the gauge. NOTE: Having the indicator within the green range is also acceptable. IFU-3393 Rev C Instructions for Use, Fenom Pro... - Page 11 The Fenom Pro will display the Stop Now screen and play an audible chime once the patient has successfully completed the breath maneuver. Results will display in 28 seconds. Press the Done button and properly dispose of the used mouthpiece.
-
Page 12: Chapter 4: Feno Measurement Preparation
Has not consumed food or fluids other than water in the preceding 60 minutes. Has not exercised or smoked in the preceding 60 minutes. When pre-test check is complete, proceed with Chapter 5: Perform FeNO Measurement. IFU-3393 Rev C Instructions for Use, Fenom Pro... -
Page 13: Chapter 5: Perform Feno Measurement
Hand the handpiece to the patient with the mouthpiece attached. Press the Start button on the test selection screen. Provide the patient with a brief overview on how to use the Fenom Pro device. Instruct patient to inhale naturally to full capacity before placing mouth on the mouthpiece. - Page 14 Remove the mouthpiece by firmly grasping around the outer diameter and twist counter-clockwise while pulling away from the handpiece. Properly dispose of the used mouthpiece. Replace the handpiece in its cradle on top of the device. IFU-3393 Rev C Instructions for Use, Fenom Pro...
-
Page 15: Chapter 6: Quality Control
Chapter 6: Quality Control The quality control (QC) measurement is performed on a Fenom Pro device by qualified operators. QC Mode is designed to ensure the instrument is operating within its specifications. QC consists of two test types: a Negative Control, and a User Control. -
Page 16: Chapter 7: Power Off Device
Hold the POWER button down for at least 1 second. Touch OK on the confirmation window. NOTE: It is recommended to keep the Fenom Pro device connected to a power supply whenever possible when powered on. Chapter 8: Practice Mode Practice mode is an option for use by a new patient in order to demonstrate the steps for performing a FeNO test. -
Page 17: Chapter 9: Device Setup
The Device Setup screen will display after powering on. From this screen, set the following device settings: Select Language Set Time/Date Add Tests These settings can be accessed and changed at any time. The Fenom Pro is now ready to begin a test. IFU-3393 Rev C Instructions for Use, Fenom Pro... -
Page 18: Configuration Settings
Touch the Time Zone drop-down list and select the correct time zone. Language Touch Language on the Settings screen. Select the desired language. A check mark next to the language indicates the selected language. IFU-3393 Rev C Instructions for Use, Fenom Pro... -
Page 19: System Information
Fenom Pro. Enter the code provided by the support representative and press Add Tests. Quality Control Status Touch Quality Control Status on the Settings screen. See Chapter 6 Quality Control for more instructions.. IFU-3393 Rev C Instructions for Use, Fenom Pro... -
Page 20: Chapter 10: General Care
Chapter 10: General Care Follow the recommendations below for cleaning and general care of the Fenom Pro and its accessories. IMPORTANT! Never attempt to open or service the Fenom Pro device or component. Operating Conditions Ensure stable operating conditions by avoiding placement of the device in direct sunlight, near sources radiating heat, or ventilation. -
Page 21: Preventive Inspections
Limited Warranty Spirosure, Inc. warrants the Fenom Pro to be free of defects in materials and workmanship for a period of 18 months from the date of shipment. Spirosure’s sole obligation under this warranty is limited to repairing or replacing, at its choice, any item covered under this warranty when such an item is returned intact and prepaid, to Spirosure or the local representative. -
Page 22: Chapter 11: Troubleshooting
Chapter 11: Troubleshooting The Fenom Pro device, subcomponents, and accessories are not field serviceable. Support Please contact Spirosure support if the Fenom Pro presents any problems that cannot be solved with the actions stated in this manual. Add Tests The Fenom Pro requires licensed tests in order to perform FeNO measurements. When the number of licensed tests approaches zero, the Licensed Tests Status Button will turn red. -
Page 23: Error And Codes
Plug device into power supply before using. Please plug in the device. Warning that the device sensor 10-065 has reached the expiration date. Please contact customer support to continue using this device. FeNO tests are disabled. IFU-3393 Rev C Instructions for Use, Fenom Pro... - Page 24 Please contact 45-048 Contact Technical Support. customer support. Issue: Battery Communication Failure. A failure has occurred on the device hardware. Please contact 45-050 support. Issue: Calibration Contact Technical Support. EEPROM Communication Failure. IFU-3393 Rev C Instructions for Use, Fenom Pro...
- Page 25 If problem persists, contact Technical Support. persists please restart the device. A failure has occurred on the 90-040 device license. FeNO tests are Please contact customer support to continue using this device. disabled. IFU-3393 Rev C Instructions for Use, Fenom Pro...
-
Page 26: Chapter 12: Technical Data
Device power consumption: < 20 VA Power supply mains voltage: 100–240 V ~50–60 Hz Exhaled NO Performance The Fenom Pro is verified to fulfill performance herein under temperature range of 15-30°C (59-86°F), relative humidity of 20-80%, and pressure range of 106-80 kPa. Linearity Slope 1.00 ±... -
Page 27: Chapter 13: Device Performance
Linearity Two Fenom Pro devices were tested as part of the linearity study. Nitric oxide (NO) was mixed in a balance gas of air to obtain 1 NO concentration levels between <10 and >300 ppb. The data showed that the test is linear across this range. -
Page 28: Accuracy
Limit of Detection Two Fenom Pro devices were tested using NO gas with air balance at 3 and 5 ppb. Five replicate determinations of each concentration were made. The mean and confidence limits at 3 ppb and 5 ppb support that the detection limit is below 10 ppb, supporting the claim. -
Page 29: Chapter 14: Reference
Manufacturer Keep out of rain & damp conditions Do not reuse Prescription Only In vitro diagnostic device MR Unsafe – Fenom Pro is not rated Use by YYYY-MM-DD (expiry) for use near magnetic resonance Caution, consult accompanying Catalog part number... -
Page 30: Chapter 14: Parts And Accessories
Warning! Any accessory not recommended by Spirosure, Inc. may result in loss of performance, damage to your Fenom PRO, or injury. The product warranty does not cover product failure or damage resulting from use with non-approved accessories. Spirosure, Inc. takes no responsibility for health and safety problems or other problems caused by the use of accessories not approved by Spirosure. -
Page 31: Bibliography
“An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications.” Am. J. Respir., 2011; vol. 184, pp. 602–615. Spirosure, Inc. 7020 Koll Center Pkwy, Suite 110 Pleasanton, CA 94566-3107 USA (888) 609-4839 support@spirosure.com IFU-3393 Rev C Instructions for Use, Fenom Pro...


Need help?
Do you have a question about the Fenom Pro and is the answer not in the manual?
Questions and answers