Subscribe to Our Youtube Channel
Summary of Contents for Medset PADSY TELESMART-H/P
- Page 1 I N S T R U C T I O N S F O R U S E English TELESMART-H/P Holter-ECG-Recording System Version D We have sound ideas.
- Page 2 All rights, particularly translation rights, to the text and illustrations are reserved. All manner of reproduction is permitted only with the approval of Medset Medizintechnik GmbH. All and any reprinting, including extracts, and all and any reproduction of the illustrations, including in a modified state, are prohibited.
- Page 3 Version 5 Revised 2013-07 GBA-TS_HP-D-EN.odt Medset Medizintechnik GmbH © Medset, PADSY, CARDIOLIGHT, SCANLIGHT, Spirosound, Ergotop are registered trade marks of Medset Medizintechnik GmbH. All other trade marks are the property of their respective owners. Medset Medizintechnik GmbH Curslacker Neuer Deich 66...
- Page 4 General Information The PADSY-Holter Holter-ECG Recording system bears the CE mark: According to directive 93/42/EEC of the Council with respect to medical products, and complies with the fundamental requirements of Appendix I of this Directive. The CE mark includes only those parts listed in the section "Accessories" and in the CE Declaration of Conformity.
- Page 5 General Information Products marked with this symbol must not be treated as normal household waste. The number printed next to this symbol corresponds to the product‘s serial number.
-
Page 6: Table Of Contents
Seite 6 Contents 1 Application and Function..................8 1.1 General....................8 1.1.1 Variants..................8 1.2 Safety....................9 2 Controls and Components...................12 2.1 Recorder ....................12 2.2 Marker and menu keys................12 2.3 Optical and acoustic signals..............13 3 Equipment startup.................... 14 3.1 Special hints for setting up the device with MacOS X ........14 4 Operation...................... - Page 7 Page 7 9.1 Accessories................... 40 9.2 Consumables..................40 TELESMART-H/P - Holter-ECG-Recording System - version D...
-
Page 8: Application And Function
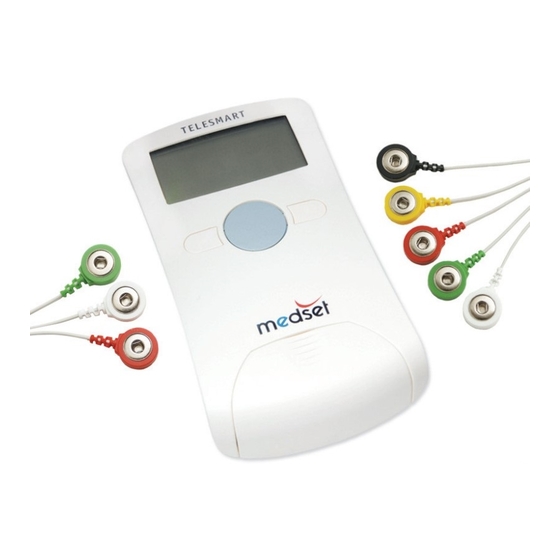
Seite 8 Application and Function Application and Function 1.1 General The PADSY-Holter Holter-ECG system consists of a TELESMART recorder with a Memory card, a recorder bag, a colour-coded patient cable to allow connection of individual ECG channels, and the PADSY-Holter evaluation software. The TELESMART-H recorder is used for ambulance or stationary recording of 2 to 3 channel Holter-ECGs. -
Page 9: Safety
Magnetic and electrical fields can influence the recorder function. Ensure that during operation, all non-Medset equipment operating in the vicinity complies with the relevant EMC requirements. This equipment may not be operated around x-ray equipment, tomographs, etc., because these devices are may emit a higher level of electromagnetic interference, corrupting the measurements. - Page 10 Liquids must never penetrate the interior of electrical equipment. If liquid has entered the recorder, it must be returned to Medset customer service for testing before it is used again. Dispose of packaging material appropriately. Ensure that it is out of reach of children.
- Page 11 Medset for replacement and made available to the user, in order to ensure safe operation. This also includes information as given on the name plate of the TELESMART recorder.
-
Page 12: Controls And Components
Seite 12 Controls and Components Controls and Components 2.1 Recorder LCD display Marker key Menu key Battery compartment Plug socket and patient cable 2.2 Marker and menu keys These keys are found on the recorder faceplate, and allow menu navigation. Marker Key The marker key is the large button below the LCD display on the TELESMART-H recorder faceplate. -
Page 13: Optical And Acoustic Signals
2.3 Optical and acoustic signals Optical Signals All commands and error messages that occur during recorder operation are dis- played. Acoustic signals System start (boot loader) After „Medset Logo“ appears System test successful Recorder start successful Keystroke Marker key Error message... -
Page 14: Equipment Startup
Seite 14 Equipment startup Equipment startup The TELESMART-H recorder is shipped in an operable condition, and can be started without performing any special commissioning procedures. Before the first startup, it is recommended that a brief check be performed, as described in the „Operation testing“ section. 3.1 Special hints for setting up the device with MacOS X Please note, that on newer MacOS X operating systems for any device which is set-up for Bluetooth, a connection code must be typed in. -
Page 15: Operation
A low-voltage warning will occur if the battery voltage is insufficient. Replace or recharge the batteries and insert the fresh batteries into the recorder. 5. After several seconds of self-test, the recorder responds with the „Medset Medizintechnik GmbH“ logo. During the system start, a progress bar may be seen at the lower edge of the screen. -
Page 16: Electrode Selection
Seite 16 Operation The preparation cream should be sparingly applied to the skin using a gauze pad at the electrode positions, and rubbed in a circular motion on the skin for approxi- mately 5 seconds. Cotton batton is not suitable, because it applies too much cream to a small area. 4.1.3 Electrode selection The TELESMART-H recorder was validated for use with the following electrodes: ARBO H92SG as standard electrodes (only suitable for 24 hour use) -
Page 17: Electrode Application
Operation Page 17 4.1.4 Electrode application To yield reproducible recordings without artefacts, the optimal application of the electrodes is necessary. Attach the electrodes to the electrode leads before they are stuck to the skin. This avoids applying hard pressure to the patient’s body. For women with large breasts, the electrodes should either be stuck to the breasts (small amplitude) or directly under them (sweat and artefacts through pressure). - Page 18 Seite 18 Operation Channel B modified V2: white (3) Left fourth intercostal space at sternal border yellow (2) Left clavicle at sternal border Ground (reference potential): black (5) right costal arch in the anterior axillary line Electrode application for patient cable 3, TELESMART-H Cable 3: red (1), yellow (2), blue (3), orange (4), green (5), white (6), black Channel A modified V4:...
- Page 19 Operation Page 19 Cable M: red (1), white (2), green (3) Channel A modified Vr5: white (2) red (1) Channel B modified V5: green (3) red (1) Channel C modified CC5: green (3) white (2) Green (3): Left fifth intercostal space in the anterior axillary line Red (1): On the sternum, level with the first intercostal space White (2):...
-
Page 20: Electrode Arrangement For Patients With Pacemakers (Telesmart-H)
Seite 20 Operation 4.2 Electrode arrangement for patients with pacemakers (TELESMART-H) Using the TELESMART recorder (TELESMART-H, only) with PADSY-Holter software al- lows the recognition and analysis of pacemaker pulses to be performed. Pulses of uni and bi polar, one and two chamber pacemakers will all be recorded and recog- nized. -
Page 21: Patients With Bipolar Pacemakers
Operation Page 21 4.2.2 Patients with bipolar pacemakers For bipolar pacemakers, the electrode array is set up almost identically to the standard configuration. To accommodate the bipolar pacemaker, the electrodes of channel B are always applied in the direction of the electrical heart axis above and below the heart. The channel B electrode separation must be shortened for bipolar pacemaker patients however. -
Page 22: Starting An Ecg Recording
Seite 22 Operation 4.3 Starting an ECG recording The communication between the TELESMART and the PC is established using a Blue- tooth wireless module. The TELESMART recorder can start the ECG recording triggered by the PC, or directly without using the PC. 4.3.1 Recorder direct start procedure When the recorder is turned on, the entry menu is displayed: Transferred recording... -
Page 23: Starting The Recorder With Padsy
Operation Page 23 existant? Back The [back] button returns to the entry menu, the [yes] and [no] buttons lead to system controls: The following appears: Back Start [Back] returns to the entry menu. The [[A] ► ►] button allows the user to switch between channels. - Page 24 Seite 24 Operation Connect with: Computer ECG Computer Doctor Back Connect The menu button [ ] allows the user to select the computer that is to be con- nected with the recorder. If the correct computer alias was selected, a connection can be established using the [connect] button.
-
Page 25: External Start
Operation Page 25 4.3.3 External start Connect with a computer as described in the previous section. This allows an exter- nal start to be performed. 1. When the recorder assistant is ended, the following display appears on the recorder: Recorder ready Last name, first name Pacemaker: Yes Next... -
Page 26: Ending The Ecg Recording
Seite 26 Operation Please instruct your patients, that the recorder has to be carried below the clothes and to fix the recorder at patients chest using the belt. If the recorder is applied to infants or certifiably insane patients, all belts and cables have to be fixed at the body sufficiently tight in order to minimize the risk of strangulation and suffocation. -
Page 27: Using Accessories
Operation Page 27 4. Read the ECG recording from the memory card according to the PADSY-Holter Holter-ECG evaluation software user’s manual. The recording can be downloaded at the user’s discretion, bearing in mind that at the next recording start, the previous recording will be deleted. 4.7 Using Accessories 4.7.1 Disposable/Rechargeable Batteries The TELESMART recorder requires either 1x1.5 V Mignon Alkaline disposable batter-... -
Page 28: Recorder Bag
Seite 28 Operation 4.7.4 Recorder bag The recorder bag protects the recorder, as well as allows it to be comfortably car- ried. The recorder bag can be secured to the patient using the nylon strap. TELESMART-H/P - Holter-ECG-Recording System - version D... -
Page 29: Cleaning And Service
Cleaning and Service Page 29 Cleaning and Service 5.1 Cleaning and disinfection General hints All components which are in contact with the patient, should be disinfected according to the recommendations of "Desinfektionsmittel-Kommission im Verbund für Angewandte Hygiene (VAH) e. V.", DGHM. Please follow the instructions of the manufacturer of the disinfectant. -
Page 30: Patient Cable
Seite 30 Cleaning and Service products are not suitable for surface disinfection and cleaning. Please follow the instructions on the cleaning and disinfection products label. 5.1.2 Patient cable Cleaning: Plugs, electrode leads, and connecting cables can be washed with lukewarm soapwa- ter or with natural cleaner. - Page 31 Cleaning and Service Page 31 The equipment should be immediately repaired if: The equipment undergoes extreme mechanical stress • Liquid enters the equipment housing • Cables, plugs or the housing is damaged • Name plate or parts of technical documentation is damaged or unreadable •...
-
Page 32: Service Life Time
Seite 32 Service life time Service life time The expected service lifetime of your TELESMART recorder is at least 7 years. Undamaged patient cables are specified for a service lifetime of at least 1½ years. Memory cards are specified for a service lifetime of at least 2 years at daily use. Please exchange the memory card immediately, if reading errors occur during the read-in process. -
Page 33: Operation Control And Error Handling
Operation control and error handling Page 33 Operation control and error handling 7.1 Operation testing Before the first use of the TELESMART recorder, please perform the following opera- tional checks. 1. Prepare the TELESMART recorder to be tested to take an ECG recording. 2. - Page 34 Seite 34 Operation control and error handling was discarded. There is a large amount of 1. An electrode has fallen 1. Carefully replace electrode noise on the signal of a off. 2. Use only electrodes and channel. 2. Expired or incorrect materials recommended in electrodes were used, or the the user’s manual...
-
Page 35: Technical Specifications
Technical specifications Page 35 Technical specifications Classification: Medical product class IIa for measurement purposes following EG guideline 93/42/EEC Protection class: Equipment with internal power supply following EN 60601 Degree of protection: IP 52 following DIN 40050 (Recorder with carrying-case) Safety classification: BF following EN 60601 ff UMDNS No.: 12-388... - Page 36 Seite 36 Technical specifications Service lifetime expected service lifetime: 7 years Transportation conditions temperature: - 10 °C to + 55 °C relative humidity: 10 % to 95 % without condensation air pressure: 700 – 1060 kPa Storage conditions temperature: 0 °C to + 45 °C relative humidity: 10 % bis 95 % without condensation air pressure:...
-
Page 37: Emc Guidance And Manufacturer's Declaration
Technical specifications Page 37 8.1 EMC guidance and manufacturer's declaration Guidance and manufacturer’s declaration – electromagnetic emissions The TELESMART holter recorder is intended for use in the electromagnetic environment specified below. The customer or the user of the TELESMART holter recorder should assure that it is used in such an environment. - Page 38 Seite 38 Technical specifications Guidance and manufacturer’s declaration – electromagnetic immunity The TELESMART holter recorder is intended for use in the electromagnetic environment specified below. The customer or the user of the TELESMART holter recorder should assure that it is used in such an environment.
- Page 39 Technical specifications Page 39 Recommended separation distances between portable and mobile RF communications equipment and the TELESMART holter recorder The TELESMART holter recorder is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the TELESMART holter recorder can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the TELESMART holter recorder as recommended below, according to the maximum output power of the communications equipment.
-
Page 40: Accessories And Consumables
Seite 40 Accessories and consumables Accessories and consumables 9.1 Accessories Article No. Item description AAZ173032 Patient cable 2, TELESMART (2 channel, 5 pole) AAZ173033 Patient cable 3, TELESMART (3 channel, 7 pole) AAZ173031 Patient cable M, TELESMART (3 channel, 3 pole) AAZ173051 Battery recharging system AAZ173001... - Page 41 Page 41 TELESMART-H/P - Holter-ECG-Recording System - version D...

Need help?
Do you have a question about the PADSY TELESMART-H/P and is the answer not in the manual?
Questions and answers