Summary of Contents for Sutter CURIS 36 0 00-01
- Page 1 INSTRUCTIONS FOR USE CURIS ® 4 MHz Radiofrequency Generator REF 36 0 00 – 01 These instructions for use apply for all devices according to chapter 5.1.
-
Page 3: Table Of Contents
CURIS Instructions for Use ® Table of contents EXPLANATION OF SYMBOLS AND ABBREVIATIONS ..............1 MODE OF ACTION AND INTENDED USE ..................2 General information about the mode of action of electrosurgery ........2 Intended use of the CURIS ....................4 ®... - Page 4 CURIS Instructions for Use ® CARE INSTRUCTIONS ........................23 Cleaning and disinfection ..................... 23 Sterilization of accessories ....................23 Accessories that cannot be sterilized .................. 23 TECHNICAL INFORMATION ......................24 Technical data, standards, certification ................24 Recurring safety-related inspections ................... 25 Diagrams ..........................
-
Page 5: Explanation Of Symbols And Abbreviations
CURIS Instructions for Use ® 1 Explanation of symbols and abbreviations Non-ionizing radiation Observe the instructions for use, notice, warning Disposal instructions Ampere Alternating current Cardiac floating Decibel Direct current Display Indicator panel on the generator Error Floating Hectopascal Hertz Kilohertz Milliampere... -
Page 6: Mode Of Action And Intended Use
CURIS Instructions for Use ® 2 Mode of action and intended use 2.1 General information about the mode of action of electrosurgery Electrosurgery is a surgical method that uses electric current to achieve surgical effects. To prevent this current from causing nerve stimulation (electric shocks), alternate current with a sufficiently high frequency (approximately 4 MHz with this device) is used so that nerve stimulation no longer occurs (Nernst equation). - Page 7 CURIS Instructions for Use ® the patient or in body recesses such as the navel or body cavities such as the vagina. Wipe up liquid that has accumulated in these areas before using the RF device. There is a risk that endogenous gases may ignite. Materials saturated with oxygen such as cotton wool and gauze may be ignited by the sparks that form during the intended use of the RF device.
-
Page 8: Intended Use Of The Curis
The instructions for use must be observed for training and application. The safe application of electrosurgery requires the user to be familiar with the technology and forms of application. Any change to the product or deviation from these instructions for use waives the liability of Sutter Medizintechnik. -
Page 9: Transportation And Packaging
3.2 Returns If possible, the original packaging must be used to return a device to Sutter or a Sutter service center. If this is not available, packaging that properly protects the device being returned is mandatory. In case of improper packaging, liability rests exclusively with the sender. The following accompanying documents must be included: ... -
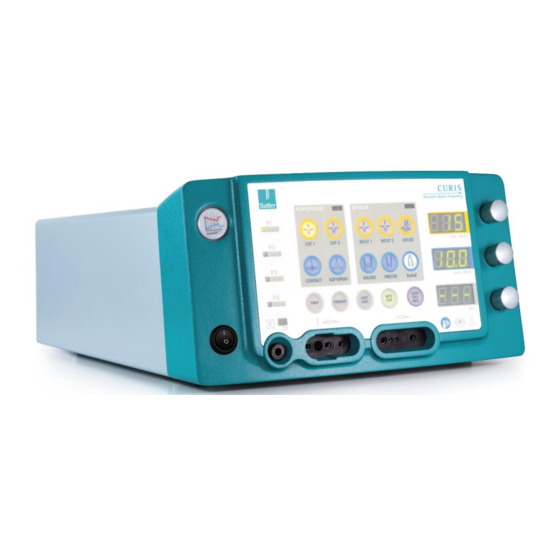
Page 10: Function And Meaning Of The Display And Control Elements
CURIS Instructions for Use ® 4 Function and meaning of the display and control elements Front of the device Mains switch “Auto-RF control active” indicator Program memory P1 … P4 (to store device settings) CONTACT selection button for monopolar contact coagulation CUT 1 selection button for monopolar cutting SOFTSPRAY selection button for contactless monopolar coagulation (spray coagulation) CUT 2 selection button for monopolar cutting with coagulation... - Page 11 CURIS Instructions for Use ® (operating modes CONTACT, SOFTSPRAY, MACRO, PRECISE, RaVoR™) Rotary knob to set the RF coagulation output Display of the TIMER time Rotary knob to set the TIMER time AUDIO FEEDBACK special function button (only possible in bipolar coagulation operating modes) Connection socket for bipolar instruments 2mm auxiliary contacts of the bipolar instrument jack AUTO STOP special function button (only possible in bipolar coagulation operating modes)
- Page 12 CURIS Instructions for Use ® Back of the device Connection socket for flushing pump Connection socket for foot switch Speaker Type plate Manufacturer Manufacturing date Safety-related inspection seal of approval Device fuse (2x T 3.15 AH 250 V G 5x20mm) Mains cable connection socket PE connection for electric potential equalization TÜV seal of approval...
-
Page 13: Commissioning
CURIS Instructions for Use ® 5 Commissioning 5.1 Validity of these instructions for use These instructions for use are valid for all CURIS devices with hardware and software according to ® the hardware and software status (48) on the type plate equal to “0607”. The type plate is on the rear of the device. -
Page 14: Self-Test
5.6.2 Connecting monopolar handles for cutting and coagulation Connecting monopolar handles with finger switch, e.g. Sutter accessory REF 36 07 04: To use monopolar handles with finger switch, connect the connection cable to... -
Page 15: Connecting Bipolar Accessories
Instructions for Use ® Subsequently activation is realized with the finger switch on the handle. Connecting monopolar handles without finger switch, e.g. Sutter accessory REF 36 02 14: To use monopolar handles without a finger switch, connect the connection cable... -
Page 16: Operating Mode Selection
CURIS Instructions for Use ® to the instructions for use. The automatically selected current type and output have to be checked for plausibility. When instruments are detected automatically, programs (buttons P1 through P4) can only be loaded and saved within the parameters assigned to the respective instrument. 5.6.5 Operating mode selection Select the desired operating mode before using the device. - Page 17 CURIS Instructions for Use ® Control dial (19) and output display (18) for the COAG operating modes (coagulation): CONTACT, SOFTSPRAY, MACRO, PRECISE, RaVoR™ The output setting can be adjusted up to a specified maximum value. For the maximum values of the various operating modes, see the technical information in chapter 9.
-
Page 18: Operation
CURIS Instructions for Use ® 6 Operation WARNING Activating the RF current is not permitted while inserting the electrode and during electrode replacement! Risk of burns! For activation, turn on the RF current according to the previously selected operating mode by operating the switch on the handle or the foot switch. - Page 19 CURIS Instructions for Use ® Procedure: 1. Press the TIMER button (29). 2. Set the maximum on time for the RF output using the rotary knob (21). 3. Turn on the electrode. After the configured time ends, the electrode turns off automatically even if the foot or finger switch is still pressed.
-
Page 20: Program Memory
CURIS Instructions for Use ® AUDIO FEEDBACK (22) This special function is only available in the BIPOLAR coagulation operating modes. During cutting and coagulation, the device produces a tone with a pitch that indicates the electric tissue resistance. The pitch of the message tone rises with the resistance as the tissue dries out more. 6.2 Program memory The device has four program memory slots on the buttons P1 …... - Page 21 CURIS Instructions for Use ® 2. Connect the plug of the connection cable for the neutral electrode to the connection socket (30) again. If a monopolar operating mode is selected, the red warning lamp (31) stops flashing now. If a segmented neutral electrode is used, it must be correctly applied to the patient for the alarm to turn off.
-
Page 22: Safety Measures
CURIS Instructions for Use ® 7 Safety measures 7.1 General information RF devices are high-frequency generators that generate high voltages and currents for their intended application. To avoid danger for the patient, operating personnel, or third parties, the device must always be used carefully and strict compliance with the operating and safety instructions is required! Ensure that disinfectants are fully removed or evaporated before using the device. -
Page 23: Pacemaker, Implants
CURIS Instructions for Use ® The neutral electrode has to be applied as close as possible to the surgical area, reliably, and with its entire surface in contact with the patient’s body. Secure contact of the neutral electrode must be ensured for the entire RF application duration. The circulation must not be impaired by the application of the neutral electrode. -
Page 24: Setting Down Rf Instruments
CURIS Instructions for Use ® or its electrodes. Carefully follow the instructions for use, such as the application of the neutral electrode! NOTE Use bipolar technology if possible. 7.5 Setting down RF instruments Do not set down any electrosurgery instruments on the patient during application pauses. -
Page 25: Accessories
CURIS Instructions for Use ® 7.7 Accessories Sutter Medizintechnik recommends the following tested accessories: Bipolar cable (REF 37 01 54 L/R/G/A/S/P) Monopolar cable with 4 mm instrument plug (REF 36 01 87) Monopolar electrode handle with finger switch for coagulation and cutting (REF 36 07 04) ... -
Page 26: Risks Due To High Electric Voltage
CURIS Instructions for Use ® NOTE For safety reasons, leaving the output setting for a bipolar cutting operating mode at “0” is recommended if using this operating mode is not intended. 7.9 Risks due to high electric voltage The application of a current type with a high voltage, in particular a monopolar high-voltage coagulation current type, can cause neuromuscular stimulation on the patient. -
Page 27: Care Instructions
CURIS Instructions for Use ® 8 Care instructions 8.1 Cleaning and disinfection Disconnect the device from the mains network for cleaning and disinfection. Liquids must not be allowed to get into the interior of the device when applying or spraying cleaning agents and disinfectants. -
Page 28: Technical Information
CURIS Instructions for Use ® 9 Technical information 9.1 Technical data, standards, certification Mains supply 100-240 V; 50/60 Hz Power consumption without RF output approx. 50 VA at max. output power approx. 500 VA Protection class IP-Code IP20 MPG classification II b LF and RF leakage according to IEC 60601-2-2... -
Page 29: Recurring Safety-Related Inspections
The following inspections must be carried out on this device at least every 24 months according to the requirements of IEC 62353. They must be carried out by Sutter or persons/organizations authorized by Sutter that, based on their education, knowledge, and experience gained through practical activity, are able to perform such safety-related inspections properly and who are not subject to any directives in regards to this inspection activity. -
Page 30: Diagrams
CURIS Instructions for Use ® NOTE We can provide you with instructions for the safety-related inspections and a service manual on request. These assist persons or companies authorized by us with the maintenance/repair of the CURIS . The service manual also lists all assemblies that ®... - Page 31 CURIS Instructions for Use ® BIPOLAR BICUT 1 BICUT 2 R (Ω) R (Ω) EXCISE MACRO R (Ω) R (Ω) PRECISE RaVoR R (Ω) R (Ω) Curve 1: Output setting “maximum output” Curve 2: Output setting “half output”...
-
Page 32: Rf Voltage
CURIS Instructions for Use ® 9.3.2 RF voltage MONOPOLAR CUT 1 CUT 2 Display (watts) Display (watts) CONTACT SOFTSPRA Display (watts) Display (watts) - Page 33 CURIS Instructions for Use ® BIPOLAR BICUT 1 BICUT 2 Display (watts) Display (watts) EXCISE MACRO Display (watts) Display (watts) PRECISE RaVoR Display (watts) Display (watts)
-
Page 34: Control Characteristic Curve
CURIS Instructions for Use ® 9.3.3 Control characteristic curve MONOPOLAR CUT MONOPOLAR COAG Display (watts) Display (watts) 1 = CUT 1 on 600 1 = CONTACT on 400 2 = CUT 2 on 600 2 = SOFTSPRAY on 600 BIPOLAR CUT BIPOLAR COAG Display (watts) -
Page 35: Guidelines And Manufacturer Declaration For Electromagnetic Compatibility
CURIS Instructions for Use ® 9.4 Guidelines and manufacturer declaration for electromagnetic compatibility Guidelines and manufacturer declaration according to IEC 60601-1-2:2014, section 7: Electromagnetic emissions The CURIS is intended for operation in an electromagnetic environment as specified below. The ® customer or user of the CURIS must ensure that it is operated in such an environment. - Page 36 CURIS Instructions for Use ® Guidelines and manufacturer declaration according to IEC 60601-1-2:2014, section 8.9: Electromagnetic emissions The CURIS is intended for operation in an electromagnetic environment as specified below. The ® customer or user of the CURIS must ensure that it is operated in such an environment. ®...
- Page 37 CURIS Instructions for Use ® Guidelines and manufacturer declaration according to IEC 60601-1-2:2014, section 8.9: Electromagnetic interference resistance The CURIS is intended for operation in an electromagnetic environment as specified below. The ® customer or user of the CURIS must ensure that it is operated in such an environment. ®...
- Page 38 CURIS Instructions for Use ® Recommended separation distances between portable and mobile HF telecommunications devices and the CURIS according to IEC 60601-1-2:2014, section 8.10 ® Specification for high-frequency wireless communication equipment Frequency Test frequency Compliance level Distance Modulation band (MHz) (MHz) (V/m) Pulse modulation...
-
Page 39: Environmental Notices
CURIS Instructions for Use ® 10 Environmental notices 10.1 Packaging The entire packaging can be returned to the seller and is recycled as far as possible. Otherwise, dispose of the packaging as waste paper and/or household garbage. 10.2 Environmentally friendly device operation During the vaporization of tissue, the concentrated, longer-term inhalation of the burn-off that occurs during intended use should be avoided. -
Page 40: Error Diagnosis
CURIS Instructions for Use ® 11 Error diagnosis Error Possible cause Error rectification No mains voltage Check the mains supply The mains cable on the outlet or Check the mains cable device is not connected correctly Elements on the front stay connection or at all dark, device does not... - Page 41 CURIS Instructions for Use ® Error Meaning Cause Resolution During the power-up test, If not caused by actuation, Activating a finger switch the device recognized disconnect the hand piece before the power-up test Err 41, actuation of the yellow and turn the device off and completes, defect in the Err 42 and/or blue finger switch of...
-
Page 42: Foot Switch (Accessory)
CURIS Instructions for Use ® 12 Foot switch (accessory) The following foot switch is offered for the CURIS ® REF 36 01 10 two-pedal foot switch without flushing pump button, explosion protected, 4 m cable Intended use The foot switch serves as a triggering mechanism of the CURIS RF generator. -
Page 43: Equipment Cart (Optional)
CURIS Instructions for Use ® 13 Equipment cart (optional) REF 36 09 00 The equipment cart designed especially for the CURIS features numerous extras such as storage ® baskets and a foot switch adapter. It is fully assembled on delivery. The CURIS is secured against sliding by engaging in the metal strip (1) on the plate of the cart and ®... - Page 44 CURIS Instructions for Use ® Notes...
- Page 45 CURIS Instructions for Use ® Notes...
- Page 48 Address of the manufacturer Distributed by: Manufacturer: Sutter Medizintechnik GmbH Tullastr. 87 79108 Freiburg / Germany Telephone: +49 (0)761 51 551-0 Fax: +49 (0)761 51 551-30 E-mail: info@sutter-med.de www.sutter-med.de Changes reserved! REF 89 91 00 - EN; 02/2019...

Need help?
Do you have a question about the CURIS 36 0 00-01 and is the answer not in the manual?
Questions and answers