Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for HLD PhysioTouch A02
- Page 1 USER MANUAL Ver. A02 SW rev 2.0x The manual contains instructions for use, maintenance and handling of the device, as well as safety precautions and warnings. Read through carefully before using the device, to ensure safety and efficiency for you and your patient. Keep for future reference.
-
Page 3: Table Of Contents
Table of Contents Safety Application specification Parts description Connectors Symbols and product markings Installation Using the PhysioTouch® - LymphaTouch® device Treatment settings Maintenance and cleaning Troubleshooting Service Software upgrade Disposal Technical data... - Page 4 Copyright© © HLD Healthy Life Devices Ltd. Helsinki, Finland. All material included in this manual is intended solely for use related to the use of the PhysioTouch® - LymphaTouch® is a trademark of HLD Healthy Life Devices Ltd. FDA device class I...
-
Page 5: Safety
Safety In this User Manual, safety precautions and warnings are indicated with the following signal words. Make sure to read and understand all safety precautions and warnings before starting to use the device. Caution – potentially hazardous situation which, if not avoided, may cause minor to moderate injuries, or damage to the device. - Page 6 • Never use the unit for purposes other than those recommended by HLD Healthy Life Devices Ltd. HLD Healthy Life Devices Ltd will not be liable for any inappropriate use of the equipment.
-
Page 7: Application Specification
Application specification Intended use: PhysioTouch® - LymphaTouch® is a negative pressure-based therapy device and aid for physiotherapy and lymphatic drainage therapy. The device is meant to be used by persons who are knowledgeable about the treatment method or under supervision of those persons. Intended conditions of use: The environment, where this device is used typically physiotherapy setting including hospitals, physiotherapy centres. - Page 8 Intended medical indication: Improvement of lymphatic circulation in the treated area, improvement of secondary lymphedema and reduction of secondary lymphedema of the Arm (SLA) Post Mastectomy. Device is used on any patient deemed to be appropriate for a physical treatment. The patient has no control of device.
-
Page 9: Parts Description
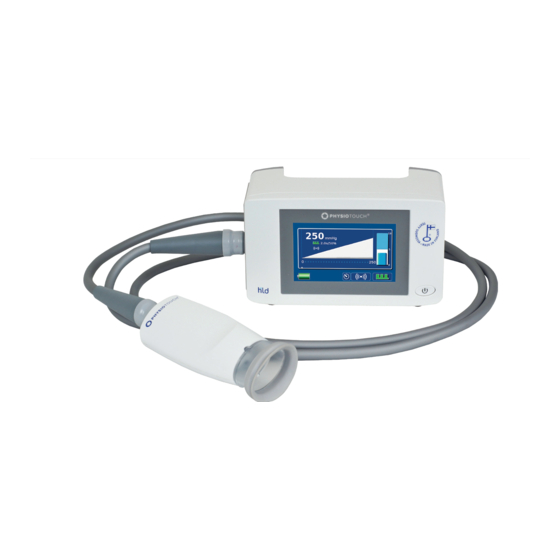
Parts description 1. Main unit 2. Handle 3. On / Off switch 4. Touch screen 5. Treatment Head 6. Cable 7. Different sizes of treatment cups for the Treatment Head 8. Soft covers for the treatment cups (detachable on the larger treatment cups) 9. -
Page 10: Connectors
Connectors 1. Device’s serial number 2. Integrated air hose and cable connector 3. Mains power 4. LAN for future use 5. USB Warning – Do not touch USB port while treating patient or let the patient touch the USB while being treated. -
Page 11: Symbols And Product Markings
Symbols and product markings In addition to the connector markers described above, you may find the following symbols and markings in the device: Symbol Description 0537 The product is CE-marked and the number indicates the notified body. Read the User Manual before using the device. The device is classified as Class BF, according to the standard IEC 60601. -
Page 12: Installation
Installation Unpacking Carefully unpack all parts of the device and confirm that all parts are included and intact. Caution - We recommend you to keep the box in case you need to return the device or send the device for service – it is most safely transported in the box it was originally shipped in. - Page 13 Connecting and disconnecting the cable Attach the integrated air hose and cable to the round connector on the left side of the unit. Make sure the cable is tightly connected, but do not use excessive force or rotate connector. To disconnect, pull the connector straight. Connecting and disconnecting the Treatment Head Attach the integrated air hose and cable to the round connector on the end of the Treatment Head.
- Page 14 Connecting a treatment cup to the Treatment Head Select the appropriate treatment cup for the patient and body area in question. Please refer to the Chapter 7.4 ”Selecting a treatment cup”. Connect the treatment cup to the Treatment Head as indicated in the figure.
- Page 15 Instructions for connecting the PhysioTouch® - LymphaTouch® main unit to the Roll Stand are included in the Roll Stand package. Caution – Use only mounting elements provided or recommended by HLD Healthy Life Devices Ltd to mount the device. If the device is not attached properly, it may fall and cause injury or damage to the equipment.
-
Page 16: Using The Physiotouch® - Lymphatouch® Device
Using the PhysioTouch® - LymphaTouch® device Turning the device on and off Turn the device on by pressing the on/off switch in the lower right hand corner. Device starts in 35 seconds. To turn off the device, press the on/off switch. The device will also shut down automatically after an hour of inactivity, while on battery power. - Page 17 Patient preparation PhysioTouch® - LymphaTouch®–treatment does not require special preparation of the patient. The treatment is applied to bare skin. Moderate application of oil or massage cream may allow the treatment cup to glide more smoothly on the skin. Avoid excessive use of oil or massage cream. If the patient’s skin is very dry and producing dandruff, the filter in the treatment cup may get clogged faster than normal.
- Page 18 Selecting a treatment cup Select the proper size treatment cup for each treatment situation. Please refer to the table below for general guidance, while using your own clinical judgment as to what is an appropriate size for each situation. Treatment cup Size Removable rubber ring Body areas •...
- Page 19 Accessories Code Accessory HLD1300 Treatment head HLD0901 Roll Stand See page 15. HLD1020-35 Treatment cup 35mm, 20pcs HLD1020-50 Treatment cup 50mm, 20pcs HLD1020-60 Treatment cup 60mm, 20pcs HLD1020-80 Treatment cup 80mm, 20pcs HLD0122 Treatment Cable HLD1601 PhysioTouch® - LymphaTouch® carrying bag Use these codes to order accessories from the nearest distributor.
-
Page 20: Treatment Settings
Treatment settings Turn on the device. PhysioTouch® - LymphaTouch® main screen will appear (see picture). The default setting is 80 mmHg with 2 s. pulsations. The unit will start operation automatically when the treatment cup is put close to the treated target. 1. - Page 21 1. The bar on the right hand side of the screen indicates the distension of the skin in the middle of the treatment cup, as a result of the applied negative pressure. 2. The battery icon in the lower left hand corner indicates the battery charge level. When the device is connected to the mains, the top of the battery icon is a power plug icon and when the battery is charging, a flash icon.
- Page 22 Pulsation screen 1. Set work/rest ratio by sliding the arrow at the top of the screen between 30 – 90%. 2. Set pulse length by sliding the arrow, or by pressing the desired location on the axis on the lower part of the screen.
- Page 23 Treatment timer PhysioTouch® - LymphaTouch® has pre-set treatment times that can be selected from the timer display. Press the treatment button to open a display where you can select the desired duration of treatment. Remaining duration of treatment is shown on the main display. See the picture below. When treatment time ends PhysioTouch®...
- Page 24 High-frequency vibration treatment feature High-frequency vibration treatment feature can be activated from the High-frequency screen by pressing the high-frequency button on the main screen. On the High-frequency screen there are two buttons; the left one is for turning the high frequency treatment off and the right one is for high frequency treatment.
- Page 25 Constant high-frequency vibration without pulsation can be set on by deactivating pulsation treatment mode and activating the high frequency treatment mode. By activating both the high-frequency vibration and the pulsation treatment mode, combination treatment can be started. Treatment mode is now vibrating pulsation treatment.
- Page 26 Numeric keypad Numeric keypad button can be found from timer setting screen. Numeric keypad –screen is used mainly in service purposes.
-
Page 27: Maintenance And Cleaning
Maintenance and cleaning Check the device for visible damage or malfunctions regularly, and replace accessories if required. Treatment cup maintenance and cleaning Wipe the treatment cups carefully using a mild disinfectant or mild detergent after each use. Disconnect the soft covers from the larger treatment cups (60 mm and 80 mm) during the cleaning process. -
Page 28: Troubleshooting
10. Troubleshooting The section below provides assistance in certain problem situations. If the suggested corrections do not fix the situation, contact device’s retailer for more instructions, or return the device for service. Problem: Device will not turn on • Battery may be empty. Connecting to mains will start charging the battery, and allow using the device with mains power immediately. - Page 29 Problem: Device is overheated • Device is used with maximum performance long period of time in hot environment. • Wait until the unit cools down and the error message disappears. Problem: Suction initiates, but the set negative pressure is not achieved. •...
-
Page 30: Service
Warning, Danger - Only persons certified by HLD Healthy Life Devices Ltd. are authorized to perform servicing of the device. Opening the device or servicing the device without authorization will result in... -
Page 31: Software Upgrade
12. Software upgrade HLD Healthy Life Devices Ltd releases new software versions to the PhysioTouch® - LymphaTouch® unit from time to time. Software can be easily upgraded by using a USB memory stick. The software upgrade is provided either via email, internet, or directly on a USB stick. More detailed instructions on performing the software upgrade and detailed description of the new software features will be included with each software upgrade. - Page 32 Proceed with the software upgrade according to the following steps: • Keep the device connected to mains during the upgrading. • Turn off the device. • Connect the memory stick to the USB connector on the left side of the device. •...
-
Page 33: Disposal
13. Disposal This is an electronic device containing a lithium-ion battery as an internal power source. Do not throw away the device with normal household waste at the end of its life. Consider the environment: deliver the device for recycling at an official collection point for electronic devices. Check with the appropriate recycling organization for local disposal information. -
Page 34: Technical Data
19 x 10 x 9,5 cm Weight Main unit 1.2 kg Power requirements Adapter 100-240 VAC, 50/60 Hz Type HLD-2200 Lithium Ion 10,8V, 5.2Ah, 56Wh Replacement interval ~3 years Duration time in use 8 hours Battery Duration time in stand by... - Page 35 10°C - 40°C, humidity 15 - 93% non-condensing, altitude 0 - 3000 m Environmental conditions Storage and transport 0° - 40° C, humidity 15 - 95% non-condensing, altitude 0 - 3000 m Medical device (CE-MDD), class 2a Classification ISO 13485:2003 Conformity to standards IEC 60601-1 IEC 60601-1-11...
-
Page 36: Emc
Warning: Use of accessories, transducers and cables other than those specified, with the exception of transducers and cables sold by HLD Healthy Life Devices as replacement parts for internal components, may result in increased electromagnetic emissions or decreased immunity of the device to such emissions. - Page 37 Emission tests according to the test specification IEC 60601-1-2. Emissions test Compliance Electromagnetic environment – guidance RF emissions Group 1 The PhysioTouch® - LymphaTouch® uses RF energy CISPR 11 only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment.
-
Page 38: Manufacturer
15.2 Guidance and manufacturer’s declaration – electromagnetic immunity The PhysioTouch® - LymphaTouch® is intended for use in the electromagnetic environment specified below. The customer or the user of the PhysioTouch® - LymphaTouch® should assure that it is used in such an environment. Portable and mobile RF communications equipment can affect medical electrical equipment. - Page 39 Immunity test IEC 60601 Compliance level Electromagnetic test level environment – guidance Surge ± 1 kV line(s) to ± 1 kV line(s) to Mains power quality IEC 61000-4-5 line(s) line(s) should be that of a typical ± 2 kV line(s) to earth ±...
- Page 40 Immunity test IEC 60601 Compliance Electromagnetic environment – guidance test level level Conducted RF 3 Vrms 3 Vrms Wireless communications equipment such as wireless IEC 61000-4-6 150 kHz – 80 MHz home network devices, mobile phones, cordless telephones and their base stations, walkie-talkies can affect this equipment and should be kept at least a distance d away from the any part of the PhysioTouch®...
- Page 41 Recommended separation distances between portable and mobile RF communications equipment and the PhysioTouch® - LymphaTouch® The PhysioTouch® - LymphaTouch® is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the PhysioTouch® - LymphaTouch® can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the PhysioTouch®...
- Page 44 HLD Healthy Life Devices Ltd. Kuortaneenkatu 2 FI - 00510 Helsinki Tel. +358 (0)10 423 7300 Fax +358 (0)10 423 7309 contact@physiotouch.com, contact@lymphatouch.com Directive 93/42/EEC www.physiotouch.com www.lymphatouch.com 0537 ISO 9001:2008 / 13485:2003 Certified company © Copyright 10/2016 v8.0 HLDID-30-1238 EN...
Need help?
Do you have a question about the PhysioTouch A02 and is the answer not in the manual?
Questions and answers