Table of Contents
Advertisement
Quick Links
1.
CONTENT OF THE PACKAGE ................................................................................................................................ 2
2.
GENERAL DESCRIPTION ....................................................................................................................................... 2
3.
CLASSIFICATION .................................................................................................................................................. 3
4.
DEVICE OPERATION ............................................................................................................................................ 3
POWER ON/OFF ............................................................................................................................................ 3
DEVICE STATUS .............................................................................................................................................. 3
5.
ENVIRONMENTAL CONDITIONS........................................................................................................................... 4
6.
DECLARATION OF CONFORMITY .......................................................................................................................... 4
7.
INTENDED USE, INTENDED USER, CONTRA-INDICATIONS AND PRECAUTIONS, RESIDUAL RISKS ............................ 4
8.
MAINTENANCE AND CLEANING ........................................................................................................................... 5
CLEANING AND STERILIZING .......................................................................................................................... 5
9.
D-HEART PORTABLE ECG HARDWARE MATERIALS ............................................................................................... 5
10. D-HEART ECG TRACING CHARACTERISTICS .......................................................................................................... 5
11. D-HEART APP ...................................................................................................................................................... 6
12. D-HEART DEVICE AND D-HEART APP COMPATIBILITY ..........................................................................................11
13. DEVICE ELECTROMAGNETIC COMPATIBILITY ......................................................................................................11
SPECIFICATIONS AND TECHNICAL INFORMATION: EMC (Electromagnetic Compatibility) .......................................12
14. DISPOSAL OF THE DEVICE ...................................................................................................................................13
D-Heart Portable ECG Device
Technical Description
Rev. 1.1.0 2018-02-14
TABLE OF CONTENTS
1
Advertisement
Table of Contents

Summary of Contents for D-Heart D-Heart
-
Page 1: Table Of Contents
D-HEART PORTABLE ECG HARDWARE MATERIALS ....................5 10. D-HEART ECG TRACING CHARACTERISTICS ......................5 11. D-HEART APP ..............................6 12. D-HEART DEVICE AND D-HEART APP COMPATIBILITY ..................11 13. DEVICE ELECTROMAGNETIC COMPATIBILITY ......................11 SPECIFICATIONS AND TECHNICAL INFORMATION: EMC (Electromagnetic Compatibility) ........12... -
Page 2: Content Of The Package
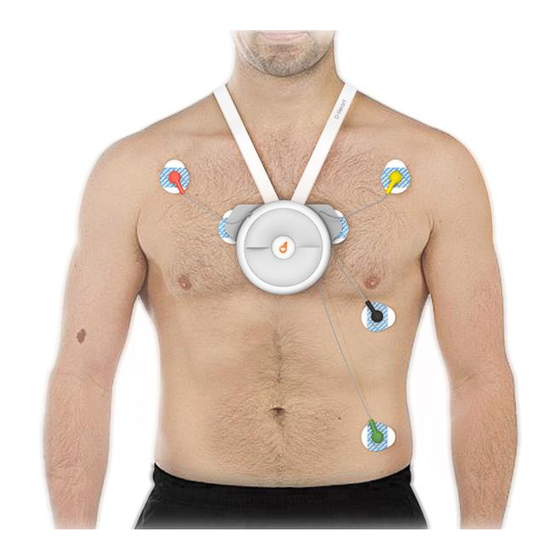
D-Heart. In order to operate, the D-Heart is connected to the human body by means of 6 sockets that are connected to disposable electrodes, glued to the skin of the body: four sockets are placed on retractable cables; two sockets are fixed in the chassis (back side). -
Page 3: Classification
3. CLASSIFICATION The D-Heart Portable ECG Device is classified as class IIa medical device according to rule 10 of Annex IX to Directive 93/42 / EEC For the purposes of the CE marking, the EC declaration of conformity for the product quality assurance system (Annex II.3 of Directive 93/42) was carried out relying on the following notified body: Notified Body no. -
Page 4: Environmental Conditions
The device is classified as Class B according to CISPR 11:2009 (Radio-frequency disturbance characteristics). • An automatic check made by the D-Heart Portable ECG Device App advices and prevents the operator from situations when the device is inoperable (e.g. not all the electrodes are connected properly to the patient’s body). -
Page 5: Maintenance And Cleaning
NEVER re-use the disposable electrodes. 9. D-HEART PORTABLE ECG HARDWARE MATERIALS D-Heart dimensions are: 117 mm x 133 mm x 44 mm D-Heart electrodes dimensions are: 31 mm x 43 mm electrodes D-Heart weight is: 196 grams D-Heart device is composed by: Battery: 601663-PLC 600mAh.doc... -
Page 6: D-Heart App
Moreover, ECG can be sent as PDF via mobile connection or printed via Bluetooth with a connected printer. 11. D-HEART APP D-Heart App offers to the user of D-Heart Portable ECG Device a full set of graphical functions described as follows. The App is available both for Android and iOS operating systems. - Page 7 The authentication controllers group is the first one that user access when the application has been launched. After a 3 seconds splash screen, D-Heart opens an infographic that explain what D-Heart is and how to use It, with a checkbox to avoid to show this controller in future.
- Page 8 The settings controller has buttons to navigate to specific application settings, device settings, last accepted Terms and Conditions and a link to the Feedback and Help system. If the user hasn’t a D-Heart device connected, there will be a button to order it. On the top part of the controller there are the number of reports available (already paid by the user) and the number of electrodes that user might have, based on the number of electrodes ordered and on the number of exams already done.
- Page 9 The patient list has a “+” button to create a new patient. If the patient will be completed by the user, the patient will be saved in the D-Heart cloud system.
- Page 10 According to FDA “Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices” and applying the following decision route, the level of concern of the software and firmware contained in the device composing the D-Heart system have been classified as: Moderate...
-
Page 11: D-Heart Device And D-Heart App Compatibility
12. D-HEART DEVICE AND D-HEART APP COMPATIBILITY D-Heart is a medical device that is regulated by international laws; we rigorously test and validate devices with smartphones and tablets to meet or exceed these regulations before we claim compatibility. We routinely add support for new phones and tablets. Check back if your device is not listed below. (Last updated... -
Page 12: Specifications And Technical Information: Emc (Electromagnetic Compatibility)
cause harmful interference to other devices, which can be determined by turning the system off and on, try to eliminate the interference by adopting one or more of the following measures: • reorient and/or relocate the receiving device; • increase the distance between the devices; •... -
Page 13: Disposal Of The Device
The old equipment can be delivered either to the reseller or to the manufacturer D-Heart srl at the time of the delivery of the new equipment. The correct disposal of waste according to the instructions above helps in preventing potential drawbacks for the environment and the public health and helps the recycling of the materials the equipment is made of.

Need help?
Do you have a question about the D-Heart and is the answer not in the manual?
Questions and answers