Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for IRIS iChemVelocity
- Page 1 1. Introduction Operator’s Manual...
-
Page 2: Table Of Contents
Tips ........................... 14 Notes ......................... 14 Cautions ........................14 Warnings ........................14 Biological Warnings ....................15 Iris Diagnostics Contact Information ................15 Manufacturer ......................15 Authorized European Representative ............... 15 Symbols .......................... 16 Warnings, Precautions, Limitations ................. 17 Precautions and Safety ....................18 Requirements ........................ - Page 3 Keyboard ......................31 Mouse ........................31 Monitor ........................ 31 Printer ........................31 Software Functions ......................32 iChemVELOCITY Status ................... 32 View Selector ......................33 Instrument Screen ..................... 33 Shift Summary ......................34 Alarms ......................... 34 Screen Level Functions ..................35 Work List ........................
- Page 4 Table of Contents Chapter 3 – Setup ........45 Settings Screen ....................... 46 Accessing a Setup Screen ..................47 Printing the Setup ...................... 47 View Log ........................48 Restore Button ....................49 Restore… Button ....................49 Save as… Button ....................49 Operator Accounts Settings ....................
- Page 5 Table of Contents Chapter 4 – Sample Processing ....75 Logon ..........................75 Specimen Preparation ..................... 76 Specimens at Room Temperature ................77 Gross Hematuria ....................... 77 Specimen Volume ..................... 77 Sample Tube Specifications .................... 78 Preparing Sample Racks ....................79 Barcode Labels ......................
- Page 6 Table of Contents Saving a Specific QC Result ................97 QC Statistics ........................99 QC Definitions ....................... 101 Mean ........................101 Standard Deviation ....................101 Coefficient of Variation .................... 101 Levey-Jennings Graph .................... 101 Target Value ......................101 Chapter 6 – Calibration Verifications ... 102 Calibration Check Material ....................
- Page 7 Table of Contents Search by date-time (range) ................120 Search by Demographics .................. 121 Show specimens awaiting transmission only ............ 123 Show released specimens only ................. 123 Show incomplete specimens only ..............123 Clear Button ...................... 123 Flagged Specimens ....................124 Review Flagged Specimen button ..............
- Page 8 Table of Contents Cleaning the Optional Load/Unload Trays .............. 138 Cleaning the Instrument Surfaces ................138 Consumable Replacement .................... 139 Weekly Maintenance ..................... 140 Cleaning the Strip Provider Module ................ 140 Monthly Maintenance ....................144 Cleaning the Wash Station Bath ................144 Cleaning the Strip Conveyor System ..............
- Page 9 Table of Contents Alarm 45 ........................164 Alarm 46 to 69 ......................164 Troubleshooting ......................165 Test Strip Jam ......................165 Strip Provider Module Jam ................165 Strip Conveyor Module Jam ................166 Rack Jam ......................166 Switching from Primary to Backup Chemistry System ..........167 Switching from Backup to Primary Chemistry System ..........
- Page 10 Table of Contents Tagging Using the Tagging Button ................188 Tagging from the Building Custom Rules Screen ........... 189 Report Generator ......................190 Search with Additional Criteria ................190 Data Management Results .................... 192 Patient Report with Tag ................... 192 Chapter 10 - Consumables Traceability .
- Page 11 Color and Clarity Report Ranges ................213 Colors ........................ 213 Clarity ........................ 213 Sensitivity and Measurement Range ................214 Analytical Sensitivity ..................214 iChemVELOCITY Urinalysis System ................215 Method Comparison ....................215 Expected Values and Ranges ................. 215 Precision ......................... 216 Within Run Precision ..................216 Day-To-Day Precision ..................
- Page 12 Table of Contents Bilirubin ........................226 Urobilinogen ......................226 Ketones ........................227 Ascorbic Acid ......................227 Glucose ........................227 Protein ........................228 Blood ........................229 pH ..........................229 Nitrite ........................229 Leukocytes ......................230 Specific Gravity ....................... 230 Color and Clarity ..................... 231 301-7146 English Rev B 11/02/2011 Operator’s Manual NA...
-
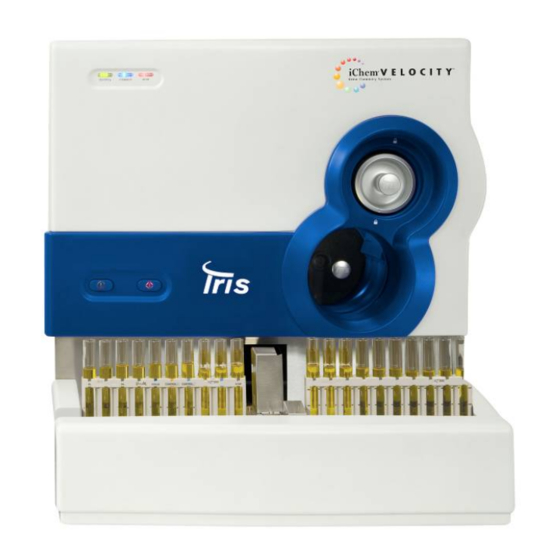
Page 13: Chapter 1 - Introduction
In particular, they are not intended for visual reading. The iChem VELOCITY test strips are not intended for visual reading. The iChemVELOCITY is not intended to be used as a Point of Care (POC) analyzer. These measurements are used to aid in the diagnosis of metabolic disorders, kidney function anomalies, urinary tract infections, and liver function. -
Page 14: How To Use The Operator's Manual
It also specifically references information concerning system components, operation, theory, utility, and performance Before operating the iChemVELOCITY, read this manual carefully. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 15: Precautions And Warnings
Cautions CAUTION: Electrical caution! Unplug before handling. CAUTION: Important information on the proper operation of the iChemVELOCITY Urine Chemistry System. This information is crucial in preventing instrument damage and maintaining the system. Warnings WARNING: Identifies potentially hazardous situations that could result in serious injury to laboratory personnel. -
Page 16: Biological Warnings
Iris Diagnostics Contact Information Customer opinion and input is extremely important to us. Iris Diagnostics wants to design products that meet your needs. Comments on this manual should be directed to:... -
Page 17: Symbols
1 Introduction Symbols The following is a list of symbols used on the product labeling consumables, the instrument, and their meaning. Symbol Meaning Caution Consult Accompanying Documents Consult Operating Instructions Control Negative Control Positive Control Date of Manufacture Do not reuse In Vitro Diagnostic Medical Device Lot Number Reference Number... -
Page 18: Warnings, Precautions, Limitations
ETL Listed Mark. Caution: Hot surface. Warnings, Precautions, Limitations • Do not place the iChemVELOCITY in water. • Do not drop or throw the instrument. • Operate the instrument on a dry, level surface. •... -
Page 19: Precautions And Safety
Connection to an inappropriate power source may cause injury or fire. Make certain that the power supply for the iChemVELOCITY is from a dedicated line that provides power to no other instruments or appliances. If power is not clean and steady, a UPS and/or power conditioner is recommended. - Page 20 1 Introduction Do not install the system during lightning activity. For protection during lightning storms and power surges, contact your facility electrical department. For added protection of the equipment during lightning and power surges, always unplug the power cord and the LIS connection. If the instrument is not used for a long period of time, unplug the power cord and the LIS connection.
- Page 21 1 Introduction WARNING: Observe Universal Precautions. Discard contaminated materials according to applicable regulations. WARNING: Dispose of waste product, unused product and contaminated packaging in compliance with applicable legal regulations. If unsure of the applicable legal requirements, contact the local authorities for information.
-
Page 22: Requirements
The instrument’s main supply inlet is being used as the mains disconnect device. I nstallation The iChem VELOCITY will be installed by a factory-trained representative from Iris Diagnostics or an authorized Distributor. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 23: Warranty
Purchaser’s claim is valid under the terms of this warranty. Iris Diagnostics makes no warranty other than the one set forth herein or that which may be provided in a separate warranty covering the applicable product category. Such limited warranty is in lieu of all other... -
Page 24: Chapter 2 - System Description
Visual measurements are no longer necessary. The iChemVELOCITY has its own power supply. A barcode reader is used to identify specimens, control and calibration material. Motors drive mechanical portions of the system. The instrument aspirates the sample and sends it to the CCGM and then dispenses the sample on each pad. -
Page 25: Color And Clarity
2 System Description Specific gravity is not measured by using a strip pad. A small flowcell is attached to a SG meter and the raw video from the linear photodiode array inside the device extends the measurement range to 1.060. Color and Clarity A Color and Clarity Module measures the color and clarity of a sample aspirated by the instrument. -
Page 26: Load And Unload Stations
2 System Description Load and Unload Stations Six (6) test tube racks (maximum of 60 test tubes) can be placed onto the load station for measurement. After sampling is completed, the test tube racks are transferred to the unload station for removal. Optional Load and Unload Stations When additional load and unload stations are connected via serial connections to the system, fifteen (15) additional test tube racks can be... -
Page 27: Specific Gravity, Color, And Clarity Module
2 System Description At the reading positions, a CMOS camera captures images of all twelve (12) pads of each chemistry strip successively illuminated with a red, green and blue light. The test strips are illuminated at each reading position with a sequence of three different LED wavelengths for less than one (1) second. -
Page 28: Fluidics
Liquid Waste Disposal Waste fluids are discarded into a drain or a waste container. On Button button is located on the left of the iChemVELOCITY. Pressing this button after applying power to the instrument, places the system in Standby. 301-7146 English Rev B 11/02/2011... -
Page 29: System Status Lights
2 System Description System Status Lights The system status lights display “Stand By” (Green), “Measure” (Blue) and “Error” (Red). Green LED only is ON The iChem VELOCITY is in Standby mode and ready for operation. The main power switch has been turned on and the ON button (front door) has been pressed. -
Page 30: 7 0 B G Reen - Blue - Red Led's Blink Synchronously
The red LED will blink when the system is waiting to connect to the user interface software. In this state, the heater is not on. The door can be opened and closed. Racks Four types of racks are used with the iChemVELOCITY system: • sample rack, •... -
Page 31: Test Strips
VELOCITY Urine Chemistry Strips are packaged in the Starter Kit with the iChem VELOCITY system. Additional strips may be purchased from Iris Diagnostics or your authorized distributor. Only iChem VELOCITY Urine Chemistry Strips may be used. Other manufacturer strips cannot be read by the instrument. -
Page 32: Peripherals
2 System Description Peripherals Keyboard The keyboard is used to enter alphanumeric specimen identifiers and data input. Mouse The mouse is used to navigate the software screens. Monitor The monitor can be used to make selections on the software screens. Printer An external USB port allows the connection of a Windows compatible printer. -
Page 33: Software Functions
2 System Description Software Functions The software provides the following functions: • Controls the mechanical functions of the iChemVELOCITY for specimen transport and fluid handling. • Automatically computes chemistry analyte concentrations, specific gravity, color and clarity results for each specimen and the results are auto-reported, unless a system flag is present. -
Page 34: View Selector
Instrument screen Instrument Screen Instrument screen is accessed by clicking on the Instrument button and is composed of the following elements: View Selector Area iChemVELOCITY Status Area Task Level Information Area Screen Level Functions Area Information Pane 301-7146 English Rev B 11/02/2011... -
Page 35: Shift Summary
2 System Description Shift Summary Field Display Button Operator Currently logged on operator if Logon any. Logoff Last Date/time of the last successful Reflectance reflectance calibration check. Check Last Date/time of the last successful SG/Color/Clarity specific gravity, color, and clarity Check calibration check. -
Page 36: Screen Level Functions
2 System Description Screen Level Functions Button Function Manual Orders Access the Manual Orders screen, see Manual Orders Consumables Access the Consumables window; see ConsumablesTraceability for more information. Quality Review Access the QC Review screen, see Reviewing Quality Controls Results QC Statistics Access the QC Statistics screen;... -
Page 37: Work List
2 System Description Work List Work List screen contains only unreleased results and is accessed by clicking on the Work List button. The Work List displays the specimen ID, date and time at which the specimen was processed, rack and tube position numbers, and status of each specimen results. -
Page 38: Chemistry Report
2 System Description • patient demographics • date/time • specimen awaiting transmission • specimen already released. The Search button allows searching in history by ID number, operator ID, demographics, date/time or specimen awaiting transmission. The results are displayed in the "Found List." The Search button toggles between the Work List and the Found List. -
Page 39: Buttons
2 System Description Buttons Buttons Functions Delete Flagged Non-recoverable flag. The specimen results will be Specimen deleted. The cause of the flag needs to be resolved before the specimen can be run again. Review Flagged Recoverable flag. Allows the user to enter a Specimen specimen ID or clear a flag. - Page 40 2 System Description 2. Select the file to open according to the desired language. 3. Click the Open button. The selected Operator’s Manual is displayed in a separate window. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA...
-
Page 41: Accessing A Section Using The Bookmark Pane
Links are indicated in the following manner: the text is displayed in blue and bold italics. Example: see Iris Diagnostics Contact Information. 1. Select the hand tool 2. -
Page 42: Iris Logo
2 System Description Iris Logo The Iris logo is displayed only when the Work List is empty. Interface Contact Iris Diagnostics Technical Service Department or your authorized distributor for information. Result Storage A maximum of 10,000 patient results can be stored in memory. Stored results can be reprinted and/or retransmitted. -
Page 43: Technical Specifications
10-tube rack system with continuous feed Specimen Volume Minimum volume 2 mL of un-spun urine Aspiration volume approx ~1mL Test Strips iChemVELOCITY Urine Chemistry Strips Test Strip Chamber Maximum capacity of 300 strips On-board stability 5 days Test Strip Waste... - Page 44 64° F to 82° F (18° C to 28° C) Temperature 20% to 80% Relative Humidity Non condensing Control iChemVELOCITY heat output approximately 1,200 British Thermal Units or BTUs Instrument Warm-up System at operating temperature on standby within 50 minutes of powering on.
-
Page 45: Consumables Or Part Replacement
2 System Description Consumables or Part Replacement REF Number Description 800-7212 iChemVELOCITY Urine Chemistry Strips 800-7704 iChem Wash Solution 7 liters container 800-7211 IRISpec CA/CB/CC Controls 800-7703 iChem CalChek Kit 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 46: Chapter 3 - Setup
Chapter 3 – Setup This section describes the steps necessary to setup and to customize the system. The iChemVELOCITY must be initially turned on in the following order to achieve correct communication between the instrument and the computer: 1. At initial start-up or if the instrument has been turned off completely, start the iChemVELOCITY by pressing the power switch on the back of the instrument. -
Page 47: Settings Screen
3 Setup Settings Screen NOTE: Only a Manager can modify the user-defined settings. Any user can view the settings. 1. To access the Settings menu, click on the Instrument button on the top right side of the main screen. 2. Click on the Go off line button. 3. -
Page 48: Accessing A Setup Screen
3 Setup • Release • Data Management The following options are only available if an iQ 200 Series module is connected to the chemistry system: • Formed Particles • REF Override • Urine Gating • Urine Auto-Release • Urine Auto-Classify Accessing a Setup Screen To access a specific setup screen, click on the appropriate button. -
Page 49: View Log
3 Setup View Log Settings are saved in a log each time they are created, modified, or deleted. The log records the date/time and the operator that made the changes. This includes changes made to Chemistry QC such as expiration date such as expiration date made via the Instrument screen \ Consumables \ Chemistry QC screen. -
Page 50: Restore Button
3 Setup Restore Button This option can be used to restore setting change directly from the View Log. 1. From the list, select the change to restore, and then click the Restore button. The setting will be restored to its previous state. 2. - Page 51 Viewing the Saved Settings This function can be used to view the saved settings, and if necessary, transmit the settings to Iris Diagnostics or your distributor. 1. Perform a Save as… and select the Desktop for the destination, and then click the Save button.
-
Page 52: Operator Accounts Settings
3 Setup Operator Accounts Settings The Operator Accounts screen displays the list of authorized users and their privilege level (Technologist or Manager). From this screen, users can be added or deleted. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 53: Adding A New Operator
3 Setup Adding a New Operator 1. From the Operator Accounts screen, click the New button. The New Operator Account screen is displayed. NOTE: The Operator Identifier, Password, and Confirm password fields are case-sensitive. 2. Type the new operator identification in the Operator Identifier field (the following characters cannot be used: [ ] ‘... -
Page 54: Deleting An Operator
3 Setup Deleting an Operator NOTE: The currently logged in operator cannot delete his/her own user account. 1. From the Operator Accounts screen, select the operator to be deleted, and then click the Delete button. 2. The system prompts for confirmation: “Really delete the selected operator account?”... -
Page 55: Laboratory Information Settings
3 Setup Laboratory Information Settings The Laboratory Information screen displays seven lines allowing the user to enter specific laboratory information that will be displayed on reports. 1. Type the laboratory information in the order it will be displayed on reports. 2. -
Page 56: System Configuration Settings
The user has the possibility to mix conventional and qualitative chemistry settings for the Iris fully automated chemistry system. These values can be user-defined in the Chemistry Settings screen. The chemistry settings can be saved for a primary and a backup chemistry system. -
Page 57: Chemistry System Present
3 Setup 2. The Options field is enabled only if the Microscopy System Present checkbox is selected. Chemistry System Present Another chemistry system can be connected to the iChemVELOCITY ® ™ using the iChem BOOST Kit. If this kit is used, both chemistry systems must be selected and configured at the time of installation. - Page 58 3 Setup 3. Use the pulldown button to select the primary chemistry system and settings to install. 4. Click the OK button to install the chemistry settings and return to the System Configuration screen. 5. Click the OK button. 6. The system will prompt to restart the software. From the Instrument screen, click the Maintenance button, and then click the Restart button.
-
Page 59: Enter Backup Chemistry Settings
3 Setup 8. From the System Configuration screen, make sure the proper primary settings are displayed. 9. Click the Save as Primary button. 10. Click the OK button. The system will prompt to restart the software. From the Instrument screen, click the Maintenance button, and then click the Restart button. - Page 60 3 Setup 3. Use the pulldown button to select the backup chemistry system and settings to install. 4. Click the OK button to install the chemistry settings and return to the System Configuration screen. 5. Click the OK button. 6. The system will prompt to restart the software. From the Instrument screen, click the Maintenance button, and then click the Restart button.
-
Page 61: Validating The Changes
3 Setup 8. From the System Configuration screen, make sure the proper settings are displayed. 9. Click the Save as Backup button. 10. Click the OK button. The system will prompt to restart the software. From the Instrument screen, click the Maintenance button, and then click the Restart button. -
Page 62: Lis Interface Settings
3 Setup LIS Interface Settings The LIS Interface screen is used to enable LIS communication and configure the LIS communication settings. It is used also to enter the patient demographics settings. The following options are only available if an iQ Series module is connected to the chemistry system: •... -
Page 63: Communication Settings
3 Setup Communication settings Use the dropdown boxes to select the specific communication settings for the LIS. Specimen information from LIS Skip Specimen if LIS Fails If this box is checked, the specimen will not be sampled for chemistry if the communications with the LIS failed. -
Page 64: Fluid Type - Formed Particles
3 Setup 3. Click OK to validate the entries and return to the LIS Interface screen. 4. When all the settings have been entered, click OK to validate the entries and return to the Settings screen. Fluid Type – Formed Particles This option is only available if an iQ Series microscopy module is connected to the chemistry system. -
Page 65: Chemistry Settings
Specific Gravity COLOR Color Clarity Do not change any Chemistry Input Settings without consultation with Iris Clinical Support. Output may be changed as needed to reflect the usual reporting terminology of the laboratory. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 66: Editing Chemistry Settings
3 Setup Editing Chemistry Settings 1. Select the row corresponding to the chemistry to be edited and click the Edit button or double-click on the row. The Chemistry Settings screen is displayed. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... - Page 67 3 Setup 2. In the Short Name field, enter the chemistry abbreviation that will be displayed on the Specimen results screen (alphanumeric.) 3. In the Long Name field, enter the chemistry name that will appear on the final reports (alphanumeric.) 4.
-
Page 68: Editing Chemistry Values
3 Setup • If a threshold value is selected, chemistry results with values equal to or higher than the abnormal threshold will be flagged H (high). The high result and the “H” flag will be displayed in red on the screen. 7. - Page 69 Adding a value Enter the new values in the edit boxes, and then click the Add button. The new values are added to the list. Do this only with the assistance of Iris Clinical Support. 301-7146 English Rev B 11/02/2011...
-
Page 70: Validating The Changes
3 Setup NOTE: Input values must match the values received from the chemistry module. Do not change the input values without the assistance of Iris Clinical Support. Validating the changes 1. When all the changes are completed for a specific chemistry, click Next to move to the next analyte, or click OK to close the screen. -
Page 71: Qc Settings
3 Setup QC Settings The QC screen allows the user to automatically send control results to the printer, the LIS or both for Chemistry QC. 1. Select the destination for QC results to be sent automatically for Chemistry QC. 2. Click OK to validate the selection and close the screen. NOTE: Both the Printer and LIS checkboxes can be selected as automatic release destinations for results. -
Page 72: Specimen Settings
3 Setup Specimen Settings The Specimen screen allows the user to control whether barcode scan failures are flagged or not. Flag barcode scan failures 1. If checked, barcode scan failures will be flagged as ID_ERROR. 2. If unchecked, the condition will not be flagged and the specimen will be assigned an identifier consisting of underlines. -
Page 73: Report Operator [Auto]
3 Setup Report operator [auto] If checked, this option will set the username to [Auto] for results that are auto-released. If unchecked, the results will display the name of the user currently logged in when the results are auto-released. Release Settings The Release screen allows the user to select the destination for the release of results. -
Page 74: Urine Gating Settings
3 Setup Urine Gating Settings This option is only available if an iQ Series microscopy module is connected to the chemistry system. Urine Auto-Release Settings This option is only available if an iQ Series microscopy module is connected to the chemistry system. Urine Auto-Classify Settings This option is only available if an iQ Series microscopy module is connected to the chemistry system. -
Page 75: Sequence Number
3 Setup Sequence Number A sequence number is assigned to samples and functions as a counter of the number of samples that are run on the system. The sequence number is displayed on the Specimen Results screen, and is displayed and printed on the specimen report. -
Page 76: Chapter 4 - Sample Processing
4 Sample Processing Chapter 4 – Sample Processing Logon The Logon button is located on the top right side of the Instrument screen. Until someone is logged on, the Specimen, Worklist and Instrument buttons are inactive. 1. Click Log On. The Log On screen is displayed. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 77: Specimen Preparation
4 Sample Processing 2. Type the user name in the Identifier field or use the drop down browser on the right side of the field to display and select the user name. 3. Type the user password in the Password field. 4. -
Page 78: Specimens At Room Temperature
Allow all refrigerated specimens to return to room temperature before testing. Gross Hematuria Use manual methods. Specimen Volume Specimen volume for analysis by the iChemVELOCITY alone should be at least 2 mL. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 79: Sample Tube Specifications
4 Sample Processing Sample Tube Specifications Dimensions: 16mm X 100mm glass or polystyrene plastic tubes. Examples of tubes that may be used and their maximum volumes Total Tube Type of Tube Maximum Volume (mL) Allowable Volume without Spillage 15 mL Fisherbrand Borosilicate 12 mL Culture Tubes... -
Page 80: Preparing Sample Racks
4 Sample Processing Preparing Sample Racks 1. Apply barcode labels to sample tubes, placing the start of the barcode (not the barcode label), approximately ½ inch below the top of a 16x100mm tube or just below the flare of a Kova or Kova-like tube. -
Page 81: Manual Orders
4 Sample Processing Manual Orders When an LIS is not available for any reason, this function allows the user to manually enter a Work List for chemistry. Accessing Manual Orders 1. To access the Manual Orders Menu, click on Instrument on the top right side of the main screen. -
Page 82: Entering Manual Orders
The Work Order option will change to "Run". b. Fluid Type - select URN. c. Dilution - not available for the iChemVELOCITY. d. Work Order - default No Order • No Order The sample will be processed according to the user defined criteria. -
Page 83: Clearing Specimen Information
4 Sample Processing 7. The rack numbers for which a manual order has been requested will be flagged with an asterisk and highlighted by color on the Manual Orders screen and will be displayed on the Instrument screen. Clearing Specimen Information From the Manual Orders screen Clear Rack button When analysis of the last specimen for a specific rack is completed... -
Page 84: Running Samples
Sampler. 5. If the iChemVELOCITY is in Measure (blue light lit), block the sensor at the front of the Sampler (nearest to the operator), and the rack will move to the sampling position automatically. -
Page 85: Consumables
45 degree angle. 4. Load the urine chemistry strips inside the Test Strip Loader as indicated on the Test Strip Loader (Iris logo facing the back of the loader.) 5. Retract the strip loader, and then shake the loader lightly so that the chemistry strips are straight. - Page 86 4 Sample Processing 6. Insert the strip loader inside the analyzer. Rotate the strip loader to the lock position so that the chemistry strips drop inside the strip provider module. The strip provider module will tumble to place the strips in the flat position. 7.
- Page 87 4 Sample Processing 11. If samples were being processed, wait two (2) minutes to allow the instrument to process the data. 12. Turn the power off by pressing the green button located on the left side of the chemistry system. 13.
-
Page 88: Replacing Wash Solution Container And Wash Solution Filter
4 Sample Processing Replacing Wash Solution Container and Wash Solution Filter As needed or quarterly CAUTION: Wear fresh gloves when changing the Wash Solution filter. Have a supply of paper towels to catch spills and drips. The iChem Wash Solution is packaged with 4 filters. Replace the Wash Solution Filter with each bottle. -
Page 89: Consumables Part Numbers
4 Sample Processing Consumables Part Numbers REF Number Description 800-7212 iChemVELOCITY Urine Chemistry Strips 800-7704 iChem Wash Solution 7 liters container 800-7211 IRISpec CA/CB/CC Controls 800-7703 iChem CalChek Kit 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 90: Chapter 5 - Quality Control
The condition and accuracy of the system can be checked by performing Control Measurements using Urine Control CA/CB/CC (REF 800-7211). The iChemVELOCITY supports the use of three (3) control solutions (CA, CB and CC) to validate the positive responses for each urine chemistry. -
Page 91: Running Qc
5 Quality Control Running QC Items required: Urine Control CA/CB/CC (REF 800-7211), Control Rack, sample tubes, protective gloves. 1. Make sure there are enough Test Strips in the Test Strip Provider. 2. Prepare Control Tubes according to the directions on the package insert. -
Page 92: Qc Failure
5 Quality Control 10. If the results are within the allowable range, the date and time of the QC will be displayed on the Last QC field of the Instrument screen. Proceed to patient testing. 11. When Control testing is completed, the results are printed and/or transmitted to the LIS depending on the user configuration (consult Settings.) QC Failure... -
Page 93: Reviewing Quality Controls Results
5 Quality Control Reviewing Quality Controls Results 1. Click Instrument. 2. Click Quality Review. The Quality Review screen is displayed. 3. Data can be sorted by clicking on a column header. A repeat click will toggle between ascending (∆) and descending (∇) order. The default is Date/Time –... -
Page 94: Print List Button
5 Quality Control Print List button Clicking Print List will print all the QC results on the list. Re-Report button Select a row and click Re-Report. The Re-Report screen is displayed. Remove The Remove button allows a manager to remove a known human error. The user with manager status logged in at the time is documented and the reason for removal should be documented. -
Page 95: Search Button
5 Quality Control Search button The Search button allows access to the Quality Search screen. The user can search the QC database using specific criteria. Chemistry button searches for chemistry controls. Lot field Displays the control lot information. Type field Displays the control type (CA/CB/CC, Specific Gravity/Color-Clarity and Reflectance.) Status field... -
Page 96: Save Button
5 Quality Control Select the specific criteria for the Search, and then click OK to display the results. Save Button This option allows the user to save QC results for long term storage in HTML format, which can be opened and saved on virtually any computer. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 97: Qc Report
5 Quality Control QC Report 1. The QC report for the chemistry system can be reviewed by highlighting the desired ID and by clicking Re-report. 2. A screen is displayed where the QC report destination can be selected; see Re-Report button. -
Page 98: Print Button
5 Quality Control Print Button Click the Print button to send the report to the printer. Save Button This option allows to user to save QC results for long term storage in HTML format, which can be opened and saved on virtually any computer. Saving all QC Results 1. - Page 99 5 Quality Control 4. Click the Save button, a standard save Windows popup is displayed. 5. Select the destination: a. E:/ USB drive b. F:/External hard drive 6. Select the folder, and then type the name for the file to be saved. Click the Save button.
-
Page 100: Qc Statistics
5 Quality Control QC Statistics 1. Click Instrument. 2. Click QC Statistics. The QC Statistics screen is displayed. 3. To view a specific report, select the control lot number. The report is displayed. a. Chemistry QCs are plotted by QC Lot number. b. - Page 101 5 Quality Control 5. Below the L-J chart is a list of each point on the chart with its status. Printing this list on the same date every month will provide the laboratory with complete documentation of QC activity. 6. Place the cursor over a data point on the Levey-Jennings chart to display the data for that point.
-
Page 102: Qc Definitions
5 Quality Control QC Definitions Mean The arithmetic average of a set of values. A measure of central tendency of the distribution of a set of replicate results. Often abbreviated by an x with a bar over it: x. In this usage, x is the running mean of the points on the graph. -
Page 103: Chapter 6 - Calibration Verifications
6 Calibration Verifications Chapter 6 – Calibration Verifications Calibration Check Material Reflectance CalChek test strips Five (5) CalChek Strips have fixed calibration values with no lot specific data. CAUTION: CalChek strips and CalChek solutions are SINGLE USE ONLY. Using the CalChek strips again will cause the reflectance CalChek to fail. -
Page 104: Calcheks Frequency
6 Calibration Verifications CalCheks Frequency CalCheks should be performed quarterly on the iChemVELOCITY system. If the instrument was moved, a CalChek should be performed to make sure that the instrument is properly calibrated. Running CalChek Reflectance CalChek The Reflectance is the measure of LED illumination light reflected from the chemistry pads. - Page 105 6 Calibration Verifications 3. Click on the Reflectance Check button. The system will display a series of six screens. Follow the instructions on the screens. NOTE: The CalChek procedure can be stopped by pressing the Cancel button when available on a calibration check screen. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA...
- Page 106 6 Calibration Verifications 301-7146 English Rev B 11/02/2011 Operator’s Manual NA...
- Page 107 6 Calibration Verifications 4. If needed, access the test strip provider. Remove and discard all Accessing the chemistry test strips present inside the chamber; see Strip Provider Module. 5. Pull the Strip Loader out of the system. Load the CalChek strips inside the Strip Loader, and then push the Strip Loader back inside the system.
- Page 108 6 Calibration Verifications 301-7146 English Rev B 11/02/2011 Operator’s Manual NA...
- Page 109 6 Calibration Verifications Loading Urine Chemistry 7. Reload iChem Velocity Test Strips; see Strips. 8. Press Finish to return to the Chemistry strip lot information screen. Check the information related to the loaded test strips, modify if necessary. 9. The reflectance CalChek results can be reviewed in the Quality Review screen, see Reviewing Quality Controls Results.
- Page 110 6 Calibration Verifications 301-7146 English Rev B 11/02/2011 Operator’s Manual NA...
-
Page 111: Specific Gravity, Color, And Clarity Calchek
Calibration rack according to the table above making sure that the barcode labels are placed at the correct positions. 4. Load the Calibration rack onto right side of the iChemVELOCITY sampler. 5. The rack will be processed and all calculations performed automatically. -
Page 112: Calchek Failure
6 Calibration Verifications CalChek Failure Reflectance CalChek Failure 1. Repeat the run using the same lot number. 2. Repeat the run using a new lot number. 3. If the CalChek failed again, contact Technical Services or your distributor. SG/Color/Clarity CalChek Failure 1. -
Page 113: Chapter 7 - Results
7 Results Chapter 7 – Results Results Interpretation Microscopy and chemistry tests are complementary, not correlative. The standard of care regarding urinalysis usually prescribes three components: physical examination, chemical examination and microscopic examination (Brunzel & Berry abstract, and AAFP 2005 MAR 15: 71(6), p1153). -
Page 114: Reviewing Results
7 Results Reviewing Results Unreleased results can be reviewed using the Work List Screen. Released results can be reviewed using the Found List Screen after a search has been performed. Work List Screen To display the Work List, click on the Work List button located on the top right part of the screen. -
Page 115: Found List Screen
7 Results Found List Screen The following descriptions apply to the Work List and the Found List. • Specimen ID • Date/Time of analysis • Rack number and Tube position • Specimen status • Status To review a specimen, double click the specimen row or select the row and click the Specimens button. -
Page 116: Delete Specimen
7 Results 1. Click the Sort Work List button. The Sort Work List screen is displayed. 2. Select the primary sort criteria. 3. Press OK. The screen closes. The Work List displays the sorted results. The ascending sort order for the Status column is: •... -
Page 117: Un-Delete Specimen
7 Results 2. A pop-up box appears prompting you to confirm the deletion. Click Yes. 3. The specimen results are removed from the Work List. They are transferred to the Delete list where they are stored until the system deletes them on a first in first out basis. Un-Delete Specimen This function allows the user to restore deleted specimen results to the Work List. -
Page 118: Correct Specimen Id
7 Results Correct Specimen ID This function allows a manager to correct a specimen ID that did not have an ID error. Any logged-in user can correct the ID of a specimen with an ID error. Edit Demographics If a specific demographic is missing (see Obtain Patient Demographics Information from LIS), the specimen will be processed and the result will... -
Page 119: Print List
7 Results Print List Clicking the Print List button prints the entire Work List. Re-Report The Re-Report function, enabled on the Found List only, allows the user to select a destination to re-report specimen results already released. The following options can be selected: •... -
Page 120: Search
7 Results 3. Select the Current Row option. If more than one row is desired, in the All Rows field, select the specific option for the re-report, Individual, or Consolidated. 4. Select the re-report destination (more than one destination can be selected.) 5. -
Page 121: Search By Sequence Number
7 Results Search by Sequence Number 1. To search specimens within a sequence range: Enter the first specimen sequence # in the From edit box. • • Enter the last specimen sequence # in the To edit box. • Click OK. 2. -
Page 122: Search By Demographics
7 Results 1. Check the “Date-time” check box. 2. To search specimens within a range: • Enter the date or click on the down arrow and select a date from the calendar. • Click on the hour and use the arrows to change. Minutes and seconds can be done the same way. - Page 123 7 Results • Click OK. 1. To search for a specific age: • Enter the age in the first edit box. To search for a specific age, the age must be reflected as a decimal value (i.e. 10 years 3 months = 10.25 yrs;...
-
Page 124: Show Specimens Awaiting Transmission Only
7 Results • When all the necessary data have been entered, click OK Show specimens awaiting transmission only This option can be combined with the other search options. 1. Click the checkbox to restrict the search to results pending transmission. 2. -
Page 125: Flagged Specimens
7 Results Flagged Specimens On the Work List screen, flags will be indicated in the Status column. To view the flag condition, double-click on the flagged specimen’s row. The Specimens screen is displayed and the flags are indicated. Information Pane Flag Flags appear in the right side of the Specimen screen. -
Page 126: Review Flagged Specimen Button
CHEMTRANSLATE One or more chemistry names or result received from the iChemVELOCITY do not match the expected name or result (data entered in the input settings). DO NOT change chemistry input values without assistance from Iris Clinical Support or your authorized distributor. -
Page 127: Id_Error
7 Results 3. The confirmation results may be added in the Edit Comment box, if desired. ID_Error The barcode reader was not able to read the barcode label. Remedies 1. From the Work List screen, double-click on the specimen to open the Specimen Review screen. -
Page 128: Processing Not Complete
7 Results 2. Refill the sample tube(s) with at least 2.0 mL of specimen. 3. Re-run the specimen(s). Processing not complete The system has detected an internal irrecoverable error. The instrument stops processing new specimens and raises an audible and/or visual alarm to signal a problem, and displays a message indicating that an unrecoverable error has occurred. -
Page 129: Specimen Screen
7 Results Specimen Screen Accessing the Specimen Screen From the Work List screen, double-click on the specimen’s row or select the specimen and then click the Specimen button. The following data are included in the Chemistry report: • Specimen ID and Patient Demographics (if enabled). •... -
Page 130: Specimen Screen Buttons
7 Results at which this happened. The most recent user is located at the top of the list. Specimen Screen Buttons Delete Flagged Specimen Delete Flagged Specimen. Review Flagged Specimen Review Flagged Specimen. Accept Click Accept to validate an option for flagged specimens. Skip Skip the displayed specimen results, and displayed the next results available on the Work List. -
Page 131: Editing Results
7 Results Editing Results In order to be able to edit Results, the function Enable Detail Audit Trail must be selected; see Chapter 3 – Specimen Settings. The operating system for the iQ 200 Series must be Windows XP. NOTE: The user ID of the operator currently logged, the date/time, and ALL changes made to the original chemistry results will be listed in the Detailed Audit Trail section of the report. - Page 132 7 Results 4. To edit a result, click the drop down button corresponding to the analyte to modify. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA...
-
Page 133: Entering Manually A Microscopy Result Value
7 Results 5. Select the new result from the drop down list. The new value is highlighted in yellow, indicating that a change was made. If necessary, check the new value for abnormal result. 6. When all results have been entered, enter a comment in the Comment field according to the Laboratory Protocol. - Page 134 7 Results 3. Click the Edit Microscopy button. The Edit Results screen is displayed. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA...
- Page 135 7 Results 4. Using the keyboard, enter the chemistry result in the New Result field corresponding to the line of the desired analyte. 5. When all results have been entered, enter a comment in the Comment field according to the Laboratory Protocol. 6.
-
Page 136: Chapter 8 - Maintenance & Service
8 Maintenance & Service Chapter 8 – Maintenance & Service Maintenance Precautions WARNING: Observe Universal Precautions. Discard contaminated materials according to applicable regulations. Daily Maintenance Items Suggested Intervals Emptying and cleaning the Waste Daily or after every 300 strips Container Discarding Liquid Waste Daily Cleaning the Sample Transport... -
Page 137: Periodic Maintenance
8 Maintenance & Service Periodic Maintenance Items Suggested Intervals Cleaning the Strip Provider Module <200 samples per day- 1 time per week. >200 samples per day- 2 times per week Perform Reflectance CalChek Quarterly, see Reflectance CalChek Specific Gravity, Perform SG/CC CalChek Quarterly, see Color, and Clarity CalChek Cleaning the Wash Station Bath... -
Page 138: Daily Maintenance
Wear protective gloves to prevent exposure to pathogens. Discard contaminated materials according to applicable regulations. Empty and Clean the Waste Container Items required: Iris System Cleanser diluted 1:10 (REF 475-0003), paper towels, and protective gloves. Empty the waste container after every 300 strips 1. -
Page 139: Cleaning The Sample Transport Module
Items required: Iris System Cleanser diluted 1:10 (REF 475-0003), lint- free tissue and protective gloves. 1. Moisten a tissue with Iris System Cleanser diluted 1:10 (REF 475- 0003) and wipe the Sample Transport Module to remove any deposits. Check under the belts and the pulleys. -
Page 140: Consumable Replacement
8 Maintenance & Service Consumable Replacement Chapter 4 for more information. NOTE: If consumables, i.e. Urine Chemistry Strips or Wash Solution need to be replaced, it can be done while the instrument is running or is in Standby. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 141: Weekly Maintenance
8 Maintenance & Service Weekly Maintenance Cleaning the Strip Provider Module NOTE: This procedure should be performed based on throughput. <200 samples per day- 1 time per week, >200 samples per day- 2 times per week. WARNING: Wear protective gloves to prevent exposure to pathogens. Discard contaminated materials according to applicable regulations. - Page 142 8 Maintenance & Service Stabilizer bar Extractor 8. Manually, rotate the black stabilizer bar to the left towards the extractor. CAUTION: Use only lint free tissues. Using a humid paper towel inside the Strip Provider Module will affect the results of the test strips. 9.
- Page 143 8 Maintenance & Service Extractor 11. Using a dry, clean brush, collect and remove the dust in the grooves between the front and the rear disks and the lower body. Collect and remove the dust where the wiper connects to the round disks. Grooves Wiper 12.
- Page 144 8 Maintenance & Service 13. Re-install the strip loader in the instrument, and turn it to the lock position. 14. To close and replace the Strip Provider Module, see Accessing the Strip Conveyor Module. 15. Close the front and left side doors. 16.
-
Page 145: Monthly Maintenance
8 Maintenance & Service Monthly Maintenance WARNING: Wear protective gloves to prevent exposure to pathogens. Discard contaminated materials according to applicable regulations. Cleaning the Wash Station Bath Items required: Deionized water, cotton swabs and protective gloves 1. Make sure the System is in Standby mode, as indicated on the top left of the instrument screen. -
Page 146: Cleaning The Strip Conveyor System
8 Maintenance & Service Cleaning the Strip Conveyor System 1. Make sure the System is in Standby mode, as indicated on the top left of the instrument screen. 2. Click on the Instrument button on the top right side of the main screen. -
Page 147: Quarterly Maintenance
8 Maintenance & Service Quarterly Maintenance Perform Reflectance CalChek Reflectance CalChek. Perform SG/CC CalChek Specific Gravity, Color, and Clarity CalChek. Replacing Wash Solution Container and Wash Solution Filter As needed or quarterly. See Replacing Wash Solution Container and Wash Solution Filter. -
Page 148: As Needed Maintenance
8 Maintenance & Service As Needed Maintenance WARNING: Wear protective gloves to prevent exposure to pathogens. Discard contaminated materials according to applicable regulations. Cleaning the Sample Tube Detector Items required: Deionized Water, cotton swabs and protective gloves. This should be performed only if the detector is missing tubes. Contact Technical Services or your authorized distributor before cleaning the window. -
Page 149: Cleaning The Optical Sensors On The Sample Transport Module
8 Maintenance & Service 4. Turn the power off by pressing the green button located on the left side of the chemistry system. 5. Open the front door to access the barcode reader. 6. Using tissue moistened with deionized water, wipe the barcode reader window. -
Page 150: Preparation For Long Term Shutdown
8 Maintenance & Service Preparation for Long Term Shutdown If the system is not used for more than a week, crystal formation may occur that can cause damage. WARNING: Wear protective gloves to prevent exposure to pathogens. Discard contaminated materials according to applicable regulations. 1. -
Page 151: Prime The System Lines
8 Maintenance & Service Prime the System Lines 1. Place three tubes in the first three positions of the Control rack. Fill each tube with Iris Diluent. 2. Run the Control rack twice to prime the internal lines. Run CalChek Running CalChek. -
Page 152: Accessing The Maintenance Menu
8 Maintenance & Service Accessing the Maintenance Menu From the Instrument screen, choose Maintenance. Maintenance options: • Reflectance Check: Allows an operator to run a Reflectance Calibration Check (see Reflectance CalChek.) • Chemistry Service: This option is reserved for Service Technicians only. -
Page 153: Auto Strip Counter Override
8 Maintenance & Service • Errors: User accessible Error Log of Chemistry Errors. • Shutdown: after going off line, allows the operator to shutdown the system. • Shutdown: This function shuts down the software and the instrument after going offline. •... -
Page 154: Restore Procedure
8 Maintenance & Service 4. Connect a blank USB media to the instrument. 5. The Backup dialog box displays the progress of the backup procedure. 6. When the backup is complete, click on the OK button to close the dialog box. Restore Procedure CAUTION: Restoring data will overwrite current data and settings. -
Page 155: System Info
8 Maintenance & Service 5. Select the option for restore, and then click the OK button. The Restore Options Confirmation dialog is displayed. NOTE: The most common use of Restore is to load Settings. For this purpose, choose "Restore instrument configuration only". If "Restore everything"... -
Page 156: Tallies
8 Maintenance & Service Tallies This option allows the user to obtain detailed information concerning the number of tests and controls run on the iQ Series system for a specific period of time. 1. From the Instrument screen, click Maintenance. 2. -
Page 157: Shutdown
3. Click the Shutdown button. The system will prompt: “Do you want to shut down the instrument?” 4. Click on the Yes button. Windows will close. 5. Turn off the iChemVELOCITY by pressing the ON button located on the left of the system. NOTE: Restart the Chemistry Module as soon as possible. -
Page 158: Restart Button
8 Maintenance & Service Restart button This button is used to shutdown and to restart only the user interface software, without any effect on the systems. This function must be used after software settings have been modified, communication settings, or error message requiring a restart of the instrument. -
Page 159: Defragment Button
8 Maintenance & Service Defragment button This function can be used when the computer performance is slow. 1. The system must be off line to perform a Defragmentation. If the system is on line, from the Instrument screen, click on the Go off line button. -
Page 160: Check Disk
8 Maintenance & Service Check Disk Some computer problems can be solved and the performance of the computer can be improved by making sure that the hard disk has no errors. Depending upon the size of the hard disk, this may take several minutes. NOTE: The Check Disk function will start only when the computer is restarted. -
Page 161: Alarm Messages
8 Maintenance & Service Alarm Messages Alarm 17 Causes Remedies The instrument is getting close to Handle specimens on the Work having too many unreleased results List, which will free some memory. in memory. Alarm 18 Causes Remedies The instrument has too many This condition must be remedied unreleased results in memory. -
Page 162: Alarm 21
8 Maintenance & Service Alarm 21 Causes Remedies One or more of the following has Restart the system. occurred: • A setting has been changed which requires restarting the system for the change to take effect. • A microscopy or chemistry system has experienced an error. -
Page 163: Alarm 37
Make sure to place each tube in the correct location and not to skip any tubes. Make sure your controls are authentic Iris controls. Make sure your controls are not expired. o Position 8: IRISpec CA™ o Position 9: IRISpec CB™... -
Page 164: Alarm 40
8 Maintenance & Service Alarm 40 Causes Remedies The Reflectance Calibration Check Repeat the Reflectance Calibration failed. Check. If it continues to fail, please call your technical support representative for further assistance. Alarm 41 Causes Remedies The SG/Color/Clarity Calibration Repeat the SG/Color/Clarity Check failed. -
Page 165: Alarm 44
8 Maintenance & Service Alarm 44 Causes Remedies The chemistry system’s waste strip Empty the chemistry system’s container is full. It becomes full in waste strip container. the normal course of running the system. Alarm 45 Causes Remedies The chemistry strip waste container Empty the chemistry system’s may be full. -
Page 166: Troubleshooting
8 Maintenance & Service Troubleshooting Test Strip Jam Strip Provider Module Jam If a jam occurs before a test strip is transferred from the Strip Provider Module to the Strip Conveyor Module, any strip that received patient sample BEFORE the jam occurred will be processed and the results will be displayed and/or released depending on the user-defined configuration. -
Page 167: Strip Conveyor Module Jam
8 Maintenance & Service Strip Conveyor Module Jam If a jam occurs on the Strip Conveyor Module, the system will stop and display a Chemistry Error message. The last reported result will be displayed indicating the rack number and the tube position. Causes Solutions Test strips are misaligned... -
Page 168: Switching From Primary To Backup Chemistry System
8 Maintenance & Service Switching from Primary to Backup Chemistry System If the primary system is not working, perform the following steps to switch to a backup chemistry system. An iChem BOOST box allows the user to enable the backup chemistry system. NOTE: Only a Manager can modify the settings. - Page 169 8 Maintenance & Service 5. From the System Configuration screen, select the backup system (for example, Other chemistry system), and then click the Install Settings button. The Select Chemistry Settings screen is displayed. 6. Use the pulldown button to select the backup chemistry system and settings.
-
Page 170: Switching From Backup To Primary Chemistry System
8 Maintenance & Service Switching from Backup to Primary Chemistry System To return the primary system back online, perform the following steps to switch to the primary chemistry system. NOTE: Only a Manager can modify the settings. Any user can view the settings. - Page 171 8 Maintenance & Service 5. From the System Configuration screen, select the primary system (for example, Iris fully automated chemistry system), and then click the Install Settings button. The Select Chemistry Settings screen is displayed. 6. Use the pulldown button to select the primary chemistry system and settings.
-
Page 172: Accessing Internal Components
8 Maintenance & Service Accessing Internal Components Accessing the Strip Provider Module 1. From the Instrument screen, click on the Go off line button. The system status will change to Off Line. 2. Press the ON button on the front of the instrument to turn the power off. - Page 173 8 Maintenance & Service 8. After cleaning inside the strip provider or removing any strip jam, close the strip loader and snugly reattach the screws. Do not over tighten. 9. Pull the blue knob to release the strip provider module and tilt it back to the left.
-
Page 174: Accessing The Strip Conveyor Module
8 Maintenance & Service Accessing the Strip Conveyor Module 1. From the Instrument screen, click on the Go off line button. The system status will change to Off Line. 2. Press the ON button on the front of the instrument to turn the power off. - Page 175 8 Maintenance & Service 7. Using the handle, gently pull the strip conveyor away from its location. 8. When the maintenance or the jam clearance is complete, replace the strip conveyor module back inside the instrument, pushing it all the way.
- Page 176 8 Maintenance & Service CAUTION: Make sure that the two tabs slide inside the two holes located at the bottom of the splashguard. If the tabs are not inside the holes, rack jam can occur. 14. Close the front door. 15.
-
Page 177: Maintenance Log
8 Maintenance & Service Maintenance Log 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 178: Chapter 9 - Iware ™ Expert System
™ 9 iWARE Expert System ™ Chapter 9 - iWARE Expert System Data Management The Data Management function uses specific filtering rules set up by the laboratory. When microscopy and/or chemistry results become available, the filtering rules are applied. Any result matching a rule can be used to signal for review by the operator. -
Page 179: Rule Generator
™ 9 iWARE Expert System Rule Generator This feature is used to create customized rules for management of results. The Rule Generator function allows the user: • To create, edit, and save logic statements for filtering results based upon multiple Urine Chemistry parameters, multiple Microscopy parameters, and patient demographics (gender, age, location). - Page 180 ™ 9 iWARE Expert System 2. Click the Go off line button. 3. A confirm window pops up (with warnings). Click Yes 4. Click on the Settings button located at the bottom of the Instrument screen. 5. The Settings screen is displayed. 6.
-
Page 181: Building Custom Rules Screen
™ 9 iWARE Expert System Building Custom Rules Screen Up to 64 numbered rules and an unlimited number of unnumbered rules can be defined using this screen. Numbered rules can be tagged, see Tagging Options. Unnumbered rules cannot be tagged and can be created to provide additional criteria to perform a customized Search. -
Page 182: Add Condition Button
™ 9 iWARE Expert System Add Condition Button Click this button to add and specify the position of a filtering condition to the selected rule. Delete Conditions Button Click this button to specify the set of conditions to be deleted from the selected rule. -
Page 183: Import Pre-Populated Rules
9 iWARE Expert System Import Pre-Populated Rules The Data Management contains rules already created by Iris Diagnostics. To utilize any of these suggested rules, the user must first import them into the system. 1. From the Building Custom Rules screen, click the Import button. -
Page 184: Creating Customized Rules
™ 9 iWARE Expert System 4. To import a specific rule, select the rule from the Pre-populated Rules field, and then click the Import button. Only one rule at the time can be selected to import. 5. To import all the available rules, click the Import All button. 6. -
Page 185: Adding One Condition
™ 9 iWARE Expert System 1. From the Building Custom Rules screen, click the Create button. 2. Use the drop-down arrow to select “Untitled”. 3. Use the drop-down arrow to display and then select the Specimen type. 4. Proceed to the condition configuration, see Adding one Condition. -
Page 186: Adding More Conditions To A Rule
™ 9 iWARE Expert System a. Use the drop-down button to select the first criteria for the rule. • Formed particles for microscopy, • Analytes for chemistry, • Last name, first name, age, gender, or location for demographics. b. Use the drop-down button to select the logic sign. The following are available: = (Equal to) <... -
Page 187: Saving A Rule
™ 9 iWARE Expert System NOTE: There is no confirmation box when deleting conditions unless all conditions will be deleted. Clicking the OK button will delete the selected conditions. Saving a Rule Click the Save button to save the selected rule after changes have been made. -
Page 188: Results/Rules Tagging
™ 9 iWARE Expert System Results/Rules Tagging This feature applies the filtering rules on each specimen results at the end of each run and takes appropriate action according to the user selection Creating a New for tagging. Only numbered rules can be tagged; see Numbered Custom Rule. -
Page 189: Info Pane Tagging
™ 9 iWARE Expert System Info Pane Tagging This selection signals in the Information pane for the operator to review the sample. Rule Matched and/or Rule Not Matched LIS Tagging This selection provides specific messages to be sent to the LIS. Rule Matched and/or Rule Not Matched Report Tagging This function incorporates reporting tags for displayed and printed... -
Page 190: Tagging From The Building Custom Rules Screen
™ 9 iWARE Expert System NOTE: Comment associated with the tagging can only be inserted from the Rule screen. 4. Check the rules for which the selected tagging will apply. 5. When tagging is completed, click the Save button. 6. Click the Rule button to return to the Building Custom Rules screen. 7. -
Page 191: Report Generator
™ 9 iWARE Expert System Report Generator This feature adds capability to create detailed, customized reports using searches from the Search Screen. Additional selection criteria derived from the Data Management rules have been added to the Search screen. Search with Additional Criteria 1. - Page 192 ™ 9 iWARE Expert System 8. Select the appropriate options and the destination, and then click the OK button. 9. Below is an example of a consolidated report obtained from data management criteria. The second line of the report indicates which rule was selected.
-
Page 193: Data Management Results
™ 9 iWARE Expert System Data Management Results Patient Report with Tag Below is an example of a patient report with a message tagged. The Rule Output field indicates the Rule Name that was applied to the results, the comment that was entered during rule tagging; the system will also indicate if the rule has been modified after the sample was run. -
Page 194: Chapter 10 - Consumables Traceability
10 Consumables Traceability Chapter 10 - Consumables Traceability Consumables Traceability This feature allows the user to keep track of the system consumables and to verify when a specific lot was in use by the system. This function provides usage tracking for the following consumables: Consumables Information iChem VELOCITY Calibration... -
Page 195: Entering Consumables Data
10 Consumables Traceability Entering Consumables Data There are two ways for the consumables data to be entered in the system: automatically using barcode labels or manual entry; see Consumables Information. In many cases, the consumables are run in barcoded tubes on special racks. -
Page 196: Chemistry Qc Consumables
10 Consumables Traceability 5. Click on the Consumables button located at the bottom of the Instrument screen. The Consumables window is displayed. Chemistry QC Consumables The Chemistry QC screens allow any user to enter strip lot information and chemistry QC such as name, lot ID, Expiration date and lower and upper limit for each analyte. - Page 197 10 Consumables Traceability 3. To access the first chemistry control level, click on Next. 4. Enter the name of the control material, lot ID, and expiration date. Select the lower and upper limits for each analyte listed. NOTE: The lower and upper limits can be selected by using the drop- down buttons only.
- Page 198 10 Consumables Traceability 9. To access the next control level, click Next. 10. After setting up all the control levels, click OK to return to the Consumables window. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA...
-
Page 199: Consumables Traceability Screen
10 Consumables Traceability Consumables Traceability Screen From the Consumables windows, click the Traceability button. The Consumables Traceability screen is displayed. The Consumables Traceability screen can also be accessed using the Search screen, see Consumables Search. Print Button Click this button to print the consumables list, see Consumables Report. -
Page 200: Add Button
10 Consumables Traceability Add Button Click this button to add a new consumable, see Adding a New Consumable for more information. Update Button Click this button to edit a consumable, see Editing an Existing Consumable for more information. In case of additions or deletions, the end dates are automatically recalculated. -
Page 201: Consumables Report
10 Consumables Traceability 3. The consumable is not removed from the list, but it will no longer be part of the date/time sequence. 4. Click the Update button. 5. Click the OK button to validate the change and close the Consumables screen. -
Page 202: Use Of Expired Material
Below is an example of red alarm for use of expired quality control material. A yellow alarm is displayed for use of expired CalChek reflectance material for the iChemVELOCITY chemistry system. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 203: Quality Control / Calibration Material
10 Consumables Traceability Quality Control / Calibration Material If expired QC or calibration material was used, a comment is added next to the run results status in the QC log or Calibration log indicating that expired Quality Control / Calibration material was used. A red alarm is raised to prevent running a sample on the instrument. -
Page 204: Chemistry Test Strips
10 Consumables Traceability Chemistry Test Strips Alarm Messages Alarm 50 Causes Remedies This alarm was triggered because This condition should be remedied the IRISpec CA™ control material by running a control rack with a is expired. new IRISpec CA™ control material. Alarm 51 Causes Remedies... -
Page 205: Alarm 52
10 Consumables Traceability Alarm 52 Causes Remedies This alarm was triggered because This condition should be remedied the IRISpec CC™ control material by running a control rack with a is expired. new IRISpec CC™ control material. Alarm 54 Causes Remedies This alarm was triggered because This condition should be remedied by running a control or patient rack... -
Page 206: Alarm 60
10 Consumables Traceability Alarm 60 Causes Remedies This alarm was triggered because This condition must be remedied the IRISpec CB™ control material for the instrument to run more is expired. specimens. • Run a control rack with a new IRISpec CB™ control material •... -
Page 207: Alarm 63
10 Consumables Traceability Alarm 63 Causes Remedies This alarm was triggered because This condition must be remedied chemistry strip is expired. for the instrument to run more specimens. • Run a control or patient rack with new chemistry strips or •... -
Page 208: Alarm 68
10 Consumables Traceability Alarm 68 Causes Remedies This alarm was triggered because This condition should be remedied Reflectance Check control material by running a control rack with a new Reflectance Check control is expired. material. Alarm 69 Causes Remedies This alarm was triggered because This condition must be remedied Reflectance Check control material for the instrument to run more... - Page 209 10 Consumables Traceability 2. Click on the Go off line button. A confirm window pops up (with warnings). Click Yes 3. Click on the Settings button located at the bottom of the Instrument screen. The Settings screen is displayed. 4. Click on the QC button, and then click OK (delete). The QC screen is displayed.
-
Page 210: Consumables Search
10 Consumables Traceability Consumables Search The Search screen allows the user to display information concerning results obtained with a specific lot of consumables. 1. From the main screen, click the Work List button. The Work List screen is displayed. 2. From the Work List screen, click the Search button. The Search screen is displayed. - Page 211 10 Consumables Traceability 5. The Search screen is displayed and the Date/Time fields during which the consumable was used is updated. 6. Click OK. 7. Results matching the search criteria are displayed in the Found List screen. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA...
-
Page 212: Chapter 11 - Appendix
11 Appendix Chapter 11 – Appendix Performance Characteristics Analytical Performance The system demonstrates an agreement > 95% within +/- 1 color block with respect to results for the same specimens run on a competitive instrument. Analytes Report Ranges (Includes pH and Specific Gravity) Conventional S. -
Page 213: Protein
11 Appendix Conventional S. I. Units Qualitative Parameter Units Units Negative Negative Negative Protein 30 mg/dL 0.3 g/L 100 mg/dL 1 g/L ≥ 500 mg/dL ≥ 5 g/L Negative Negative Negative Blood 0.03 mg/dL 0.3 mg/L (Hemoglobin) 0.2 mg/dL 2 mg/L ≥... -
Page 214: Ascorbic Acid
11 Appendix Conventional S. I. Units Qualitative Parameter Units Units Negative Negative Ascorbic Acid 20 mg/dL 40 mg/dL From 1.000 From 1.000 Specific to 1.060 to 1.060 Gravity Color and Clarity Report Ranges Colors Colorless Straw Yellow Amber Blue Clear, Slightly Cloudy, Cloudy, Turbid Clarity 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 215: Sensitivity And Measurement Range
11 Appendix Sensitivity and Measurement Range Analytical Sensitivity Analytical sensitivity is the concentration at which 67% of samples tested give a positive result. Analyte Pad Analytical Sensitivity Measuring Range Glucose 20 mg/dL 50 - 500 mg/dL Protein 20 mg/dL 30 - 500 mg/dL Bilirubin 1.8 mg/dL 2.0 -4.0 mg/dL... -
Page 216: Ichemvelocity Urinalysis System
11 Appendix iChemVELOCITY Urinalysis System Method Comparison The performance of the iChemVELOCITY was compared with commercially available urinalysis systems. Urine samples were from a hospital or a commercial laboratory. Samples with concentrations covering the entire assay reporting range were tested. The urinalysis systems used provided semi-quantitative and/or qualitative results with the exception of SG, where quantitative results were provided. -
Page 217: Precision
11 Appendix Precision Within Run Precision Three levels of target analyte concentrations for each assay (negative, first positive level and highest positive level) were tested 3 times each for 20 runs. The exact % agreement with expected results is shown. Test Analyte Concentration % Exact Agreement... -
Page 218: Day-To-Day Precision
11 Appendix Day-To-Day Precision Three levels of target analyte concentrations for each assay (negative, first positive level and highest positive level) were tested over 20 non- consecutive days. The exact % agreement with expected results is shown. Test Analyte Concentration % Exact Agreement <1 mg/dL bilirubin Bilirubin... -
Page 219: Instrument To Instrument Precision
Instrument to instrument precision Three levels of target analyte concentrations for each assay (negative, first positive level and highest positive level) were tested 60 times on each of three iChemVELOCITY systems. The exact % agreement with expected results is shown. Test... -
Page 220: Strip Lot To Strip Lot Precision
Three levels of target analyte concentrations for each assay (negative, first positive level and highest positive level) were tested 20 times on each of three iChemVELOCITY urine chemistry strip lots. The exact % agreement with expected results is shown. Test... -
Page 221: 20 Days Specific Gravity Day-To-Day Precision
11 Appendix 20 Days Specific Gravity Day-to-Day Precision Percent Coefficient Mean Standard Deviation of Variation IRISpec CA = 1.012 0.0018 0.018% IRISpec CB = 1.016 0.0009 0.009% IRISpec CC = NA* * Specific gravity is not a controlled parameter for IRISpec CC 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... -
Page 222: Day 1 - Day 20 Results For Color And Clarity
Day 1 – Day 20 Results for color and Clarity The color and clarity measurements day-to-day precision of the iChemVELOCITY over a wide dynamic range is demonstrated using the iChemVELOCITY CalChek color solutions (colorless, straw, yellow, and amber) and clarity solutions (slightly cloudy, cloudy, and turbid). -
Page 223: Clinical Concordance Tables
11 Appendix Clinical Concordance Tables Ascorbic Acid iChemVELOCITY (mg/dL) iChem 100 (mg/dL) 99.7% 95.9% 100% Exact Agreement Agreement +/- 1 100% 100% 100% Grade Bilirubin iChemVELOCITY (mg/dL) iChem 100 (mg/dL) 99.4% 100% 100% Exact Agreement Agreement +/- 1 100% 100%... -
Page 224: Glucose
11 Appendix Glucose iChemVELOCITY (mg/dL) 500 - 1000 iChem 100 (mg/dL 100% 100% 60.7%* 90.2% Exact Agreement Agreement +/- 1 100% 100% 100% 100% Grade *This discrepancy is due to the reflectance border separating 50 from 150 mg/dL. This design concept results in reduced concordance at the 50/150 border. -
Page 225: Nitrite
11 Appendix Nitrite iChemVELOCITY (mg/dL) iChem 100 (mg/dL) 100% 96.0% Exact Agreement 100% 100% Agreement +/- 1 Grade iChemVELOCITY (mg/dL) iChem 100 (mg/dL) Exact Agreement 76.9% 100% 100% 100% Agreement +/- 1 Grade 100% 100% 100% 100% 100% Protein iChemVELOCITY... -
Page 226: Urobilinogen
11 Appendix Urobilinogen iChemVELOCITY (mg/dL) iChem 100 (mg/dL) 100% Exact Agreement Agreement +/- 1 100% 100% 100% Grade Specific Gravity SG was measured using the iChemVELOCITY SG refractometer. Sample iChem 100 S.G. Avge 1.000 1.005 1.010 1.015 1.020 1.025 1.030 1.035 1.0009... -
Page 227: Principles Of The Tests And Limitations Of The Procedure
11 Appendix Principles of the Tests and Limitations of the Procedure Bilirubin This test is based on the coupling of bilirubin with diazonium salt in an acidic medium. A pinkish tan color proportional to bilirubin concentration is generated. • Expected Values: In normal urine, no detectable level of bilirubin should be obtained. -
Page 228: Ketones
11 Appendix medications that have an intrinsic red color in acidic medium such as red beets, azo dyes, phenazopyridine and p-aminobenzoic acid may produce false positive results. Prolonged exposure to light may lead to diminished or false negative values. Ketones This test is based on Legal’s method in which the test pad contains sodium nitroprusside and glycine in an alkaline medium. -
Page 229: Protein
11 Appendix hydrogen peroxide via the oxidation of glucose. Peroxidase then catalyzes the reaction of hydrogen peroxide with a chromogen via the oxidation of chromogen to colors ranging between green to gray-blue. Other sugar compounds are not detected. • Expected Values: A small amount of glucose (up to 20 mg/dL) may be present in the normal urine. -
Page 230: Blood
11 Appendix Blood This pseudo-enzymatic test contains organic peroxide and a chromogen. The peroxidase effect of hemoglobin and myoglobin causes a color change to green. • Expected Values: Normal urine contains no detectable hemoglobin or intact red blood cells. Any positive results should be further evaluated. -
Page 231: Leukocytes
11 Appendix Performance Characteristics: This test is specific for nitrite. Results • may depend on the ability of bacteria to reduce nitrate to nitrite, the number of bacteria and the retention time of the urine in the bladder. • Limitations: Food dyes and therapeutic pigments such as red beets and pyridium may cause false positive responses. -
Page 232: Color And Clarity
11 Appendix Color and Clarity Color and Clarity are measured directly from transmitted and scattered light in the sample using proprietary algorithms embedded in the Urine Chemistry System. 301-7146 English Rev B 11/02/2011 Operator’s Manual NA... - Page 233 Index Alarm 61 ............205 Alarm 63 ............206 Alarm 66 ............206 Alarm 67 ............206 1.000 ............... 127 Alarm 68 ............207 Alarm 69 ............207 Alarm Messages ........160, 203 Alarms ............... 34 20 Days Specific Gravity Day-to-Day Precision220 Analytes ............
- Page 234 Index Button, tagging ..........180 Consumable, Deleting a ........199 Button, tagging using the tagging ....188 Consumable, Editing a ........199 Button, Update ..........199 Consumables ............ 84 Buttons ............. 38 Consumables Information ......193 Consumables or Part Replacement .... 44, 88 Consumables Report ........
- Page 235 Interpretation Editing chemistry values ........67 Results ............112 Enable LIS............61 Iris Diagnostics Contact Information ....15 Entering Consumables Data ......194 Iris Logo ............41 Entering Manual Orders ........81 Entering Manually a Chemistry Result Value . 132 Expired Consumable Lockout Override ..
- Page 236 Index Modifying an Operator ........53 Mouse ............... 31 QC Definitions ..........101 QC Result, Saving a specific ......97 QC Results, Saving all ........97 Nitrite ............. 212 QC Settings ..........70, 89 Notes ..............14 QC Statistics ............99 Number, search by sequence ......
- Page 237 Index Rule, Saving a ..........186 Specific Gravity ......... 23, 213 Rules, creating populated ....... 183 Specific Gravity CalChek solutions ....102 Rules, import pre-programmed ...... 182 Specific Gravity, Color, and Clarity CalChek ..110 Rules/Results Tagging ........187 Specific Gravity, Color, and Clarity Module ..26 Run CalChek ............
- Page 238 Index Theory of Operation ......... 23 Tips ..............14 Touchscreen Monitor ........31 Validating the changes ........69 Traceability, Consumables ......193 Validating the Changes ........60 Traceability, Consumables screen ....198 Value, entering manually a chemistry result .. 132 Type field ............
Need help?
Do you have a question about the iChemVelocity and is the answer not in the manual?
Questions and answers