Advertisement
Table of Contents
- 1 Table of Contents
- 2 Safety Information - Nmes Mode
- 3 Safety Information - Tens Mode
- 4 Description of Apparatus & Controls
- 5 Description of Apparatus & Display
- 6 Step by Step Treatment Guide
- 7 System Maintenance
- 8 Troubleshooting
- 9 Technical Information
- 10 Program Information
- 11 Warranty
- Download this manual
Advertisement
Table of Contents

Summary of Contents for Neurotech avivastim xp
- Page 3 Introduction Dear Customer Thank you for choosing the AvivaStim XP™ Muscle Therapy System. Neurotech® developed AvivaStim XP as a highly portable and effective dual channel muscle stimulation device that re-educates and strengthens atrophied, weakened or immobilized muscles. Our goal is to design products that help accelerate recovery and return patients to a more active lifestyle.
- Page 4 Each AvivaStim XP unit is attributed a serial number which is located on the back of the unit. The information and technical data disclosed in this document are proprietary to Bio-Medical Research Ltd. and may only be used and disseminated for the purposes and to the extent specifically authorised in writing by the company.
-
Page 5: Table Of Contents
INDEX SAFETY INFORMATION - NMES MODE.................1 SAFETY INFORMATION - TENS MODE..................4 DESCRIPTION OF APPARATUS & CONTROLS ..............7 DESCRIPTION OF APPARATUS & DISPLAY ................ 9 STEP BY STEP TREATMENT GUIDE .................. 11 SYSTEM MAINTENANCE ....................15 TROUBLESHOOTING ......................17 TECHNICAL INFORMATION ....................18 PROGRAM INFORMATION .................... -
Page 6: Safety Information - Nmes Mode
Contraindications • Patients with electronic implants (e.g. cardiac pacemaker or defibrillator - as your neurotech product may interfere with the proper functioning of the implanted stimulator) or if you suffer from any other heart problem. - Page 7 • Stimulation should not be applied transthoracically in that the introduction of electrical current into the heart may cause cardiac arrhythmias. • Stimulation should not be applied transcerebrally. • Stimulation should not be applied over swollen, infected, or inflamed areas or skin eruptions, e.g., phlebitis, thrombophlebitis, varicose veins, etc.
- Page 8 • Electronic monitoring equipment (such as ECG monitors and ECG alarms) may not operate properly when the stimulator is in use. • It may not be appropriate to use AvivaStim XP on a person at the same time as other equipment. You should check suitability before use.
-
Page 9: Safety Information - Tens Mode
General description of AvivaStim XP for TENS AvivaStim XP also has a Transcutaneous Electrical Nerve Stimulation (TENS) program for the treatment of acute pain. Please see page 21 Program 9 for details. - Page 10 • Stimulation should not be applied transcerebrally. • Stimulation should not be applied over swollen, infected, or inflamed areas or skin eruptions, e.g., phlebitis, thrombophlebitis, varicose veins, etc. • Stimulation should not be applied over, or in proximity to, cancerous lesions. Precautions •...
- Page 11 • Electronic monitoring equipment (such as ECG monitors and ECG alarms) may not operate properly when the stimulator is in use. • It may not be appropriate to use AvivaStim XP on a person at the same time as other equipment. You should check suitability before use.
-
Page 12: Description Of Apparatus & Controls
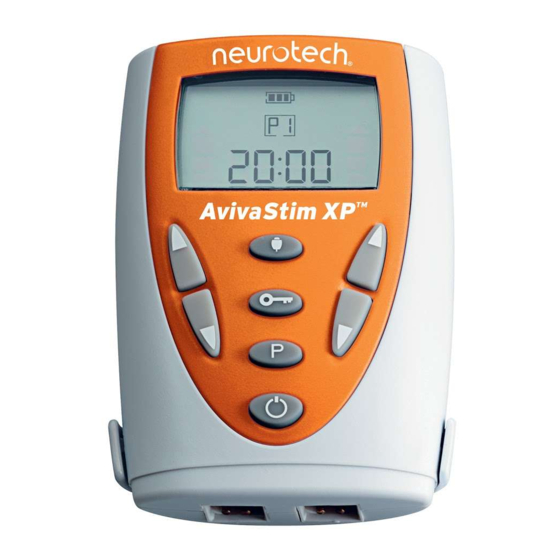
DESCRIPTION OF APPARATUS & CONTROLS LCD Display The AvivaStim XP is easy to use. All keys are controlled by push buttons. Fig. 1 The functions are defined by printed icons on each key (see below). The AvivaStim XP has a built-in audio indicator which will emit a raised tone when there is a valid key press and a low tone when an invalid key is pressed. - Page 13 4. Lock key The Lock key allows the user to lock the intensity controls preventing accidental changes in the intensity level. Reset the Total Treatment Time. The user must first press the Lock key and then the Program Select for around 3 seconds. A tone will sound and the display will reset to zero.
-
Page 14: Description Of Apparatus & Display
The unit is powered by 1 x 9-volt DC battery. The battery compartment is located on the rear of the unit. We recommend an alkaline battery. The AvivaStim XP has an indicator that shows the battery status. When the battery is nearing discharge, the battery outline will flash. To insert, replace or check the battery, follow the instructions provided on page 15. - Page 15 AvivaStim XP display (Fig. 3) The AvivaStim XP has a unique display that gives the user a precise Fig. 3 overview of the battery status, the completed treatment time, con- traction/relaxation phases and program selection. Lock key is activated and prevents unwanted changes to the intensity level.
-
Page 16: Step By Step Treatment Guide
• Once the electrodes are attached, you may separate the leads to allow for better electrode placement. • The AvivaStim XP is equipped with a belt clip. You may attach the unit at the waist by attaching it to a belt. - Page 17 6. When the AvivaStim XP is switched on you hear a high sound. The screen will display the Total Treatment Time in hours and minutes for a period of 3 seconds (Display 1). After 3 seconds the screen in Display 2 will appear on the screen.
- Page 18 12. Continue to increase the intensity until the desired level has been achieved. Where more than one channel is being used, you may increase the intensity completely from one channel before increasing the intensity from the other. Display 6 shows the screen during a contraction cycle for a timed treatment program.
- Page 19 16. The AvivaStim XP has a load sense function that monitors the connection between the cable/electrode and the user. When poor skin contact is detected: • The amplitude bar of the channel being used will flash. • The warning symbol ( ) will appear flashing on the display (Display 11).
-
Page 20: System Maintenance
Fig. 7 This means that the batteries must be exchanged. The battery compartment is located on the rear of the AvivaStim XP unit. In order to open the battery compartment turn the AvivaStim XP onto the front. Insert your thumb into the symbol shown ( ) on the battery compartment to unlock it and press it forwards. - Page 21 Repair, service and modifications may not be carried out by anyone other than qualified service personnel authorised by neurotech®. Do not use the unit if it is defective. Please return it to neurotech® or Bio-Medical Research Ltd. will not accept any responsibility where the guidelines and instructions are not followed.
-
Page 22: Troubleshooting
TROUBLESHOOTING Problem Possible Cause Solution The display does not come on & Battery discharged Replace battery there is no signal from the unit Battery was incorrectly positioned Remove battery, replace correctly Lead not fully inserted Remove plug, re-insert The unit is switched on but does not respond to commands Broken lead Replace electrode/ lead assembly... -
Page 23: Technical Information
This ensures there is no interaction between the electrodes of each channel. Electrode area less than 7.5 cm can cause current densities in excess of 2m/cm at maximum intensity. If in doubt, contact neurotech® or your clinician. - Page 24 Nominal output voltage / power Ω Ω Ω Parameter 1.5k Output RMS voltage (RMSV) 7.5 V 12.32 V 13.74 V Output RMS current (RMSA) 15 mA 12.3 mA 9.16 mA Output frequency 4-99 Hz 4-99 Hz 4-99 Hz DC Component Pulse Width 80 –...
- Page 25 Sizes: 45mm x 45mm, 50mm x 50mm, 70mm x 70mm. Pals Flex Stimulation by Axelgaard Manufacturing Company Inc. Sizes: 50mm x 50mm, 70mm x 70mm. Synapse (Medicom TENS electrodes) by Ambu A/S. Sizes: 50mm x 50mm. Leads: AvivaStim XP/ AvivaTens XP Lead (Part No.: 1600-9301). Size: Length = 1m...
-
Page 26: Program Information
PROGRAM INFORMATION NMES Programs Program Frequency Contraction Relaxation Ramp Up Ramp Down Length of Burst or Treat Time (Hz) (sec.) (sec.) (sec.) (sec.) pulse (µsec) Trigger (mins) Trigger Trigger Trigger Trigger Trigger ch1: 50 None ch2: 10 (Note 1) None (Note 2) Trigger (Note3) -
Page 27: Warranty
• has not been connected to an unsuitable power source. • has not been subjected to misuse or neglect. • has not been modified or repaired by anyone other than an approved neurotech agent. This warranty complements existing national guarantee obligations and does not affect your statutory rights as a consumer. - Page 32 Part No: XXXX-XXXX Rev.: Issued: 12/09...
Need help?
Do you have a question about the avivastim xp and is the answer not in the manual?
Questions and answers
where can we buy this?