Table of Contents
Advertisement
Instructions to User
Dear users, thank you very much for purchasing the Pulse
Oximeter.
This Manual is written and compiled in accordance with the
council directive MDD93/42/EEC for medical devices and
harmonized standards. In case of modifications and
software upgrades, the information contained in this
document is subject to change without notice.
The Manual describes, in accordance with the Pulse
Oximeter's features and requirements, main structure,
functions, specifications, correct methods for transportation,
installation, usage, operation, repair, maintenance and
storage, etc. as well as the safety procedures to protect both
the user and equipment. Refer to the respective chapters for
details.
Please read the User Manual carefully before using this
product. The User Manual which describes the operating
procedures should be followed strictly. Failure to follow the
User Manual may cause measuring abnormality, equipment
damage and human injury. The manufacturer is NOT
responsible for the safety, reliability and performance issues
and any monitoring abnormality, human injury and
equipment damage due to users' negligence of the operation
instructions. The manufacturer's warranty service does not
cover such faults.
Owing to the forthcoming renovation, the specific products
you received may not be totally in accordance with the
I
Advertisement
Table of Contents

Summary of Contents for Contec CMS50D+
- Page 1 Instructions to User Dear users, thank you very much for purchasing the Pulse Oximeter. This Manual is written and compiled in accordance with the council directive MDD93/42/EEC for medical devices and harmonized standards. In case of modifications and software upgrades, the information contained in this document is subject to change without notice.
- Page 2 description of this User Manual. We would sincerely regret for that. This product is medical device, which can be used repeatedly. WARNING: Uncomfortable or painful feeling may appear if using the device ceaselessly, especially for the microcirculation barrier patients. recommended that the sensor should not be applied to the same finger for over 2 hours.
-
Page 3: Table Of Contents
CONTENTS 1 SAFETY ..............1 1.1 I ....1 NSTRUCTIONS FOR PERATIONS 1.2 W ............1 ARNING 1.3 A ............3 TTENTION 2 OVERVIEW .............. 6 2.1 C : ..........6 LASSIFICATION 2.2 F ............7 EATURES 2.3 M AJOR PPLICATIONS AND COPE OF .............. - Page 4 7.1 C ......25 LEANING AND ISINFECTING 7.2 M ............25 AINTAIN 7.3 T ..... 25 RANSPORTATION AND TORAGE 8 TROUBLESHOOTING .......... 26 9 KEY OF SYMBOLS ..........28 10 FUNCTION SPECIFICATION ......31...
-
Page 5: Safety
1 Safety 1.1 Instructions for Safe Operations Check the main unit and all accessories periodically to make sure that there is no visible damage that may affect patient’s safety and monitoring performance about cables and transducers. It is recommended that the device should be inspected once a week at least. - Page 6 Ensure that the environment in which the device is operated is not subject to any sources of strong electromagnetic interference, such as radio transmitters, mobile telephones, etc. Keep them far away High level electromagnetic radiation emitted from such devices may greatly affect the monitor performance.
-
Page 7: Attention
Please choose the accessories and probe which are approved or manufactured by the manufacturer, or else it may damage the device. Please don't measure this device with functional tester for the device's related information. When uploading, do keep the patient form touching the USB port. - Page 8 Do not have the oximeter immerged in liquid. When it needs cleaning, please wipe its surface with medical alcohol by soft material. Do not spray any liquid on the device directly. When cleaning the device with water, the temperature should be lower than 60 ℃.
- Page 9 The hanging rope attached to the device is made from Non- allergy material, if particular group are sensitive to the hanging rope, stop using it. In addition, pay attention to the use of the hanging rope , do not wear it around the neck on the purpose of avoiding harm to the patient.
-
Page 10: Overview
2 Overview The pulse oxygen saturation is the percentage of HbO in the total Hb in the blood, so-called the O concentration in the blood. It is an important bio-parameter for the respiration. A number of diseases relating to respiratory system may cause the decrease of SpO in the blood, furthermore, some other causes such as the malfunction of human body's... -
Page 11: Features
Class II (U.S.FDA) 2.2 Features A. Operation of the product is simple and convenient. B. The product is small in volume, light in weight and convenient in carrying. C. Low power consumption 2.3 Major Applications and Scope of Application The Pulse Oximeter can be used in measuring the pulse oxygen saturation and pulse rate through finger. - Page 12 a) Temperature: 10 ℃ ~ 40 ℃ b) Relative Humidity: ≤ 75% c) Atmospheric pressure: 700 hPa ~ 1060 hPa...
-
Page 13: Principle
3 Principle Principle of the Oximeter is as follows: An experience formula of data process is established taking use of Lambert Beer Law according to Spectrum Absorption Characteristics of Reductive Hemoglobin (Hb) and Oxyhemoglobin (HbO ) in glow & near-infrared zones. Operation principle of the device is: Photoelectric Oxyhemoglobin Inspection Technology is adopted in accordance with Capacity Pulse Scanning &... -
Page 14: Technical Specifications
4 Technical Specifications 4.1 Main Performance value display Pulse rate value display, bar graph display Pulse waveform display Low-voltage indication: low-voltage indicator appears before working abnormally which is due to low-voltage Display direction can be changed automatically,easy to view. The product will enter standby mode when no signal is in the product within 5 seconds. - Page 15 B. Measurement of pulse rate Measuring range: 30 bpm ~ 250 bpm Accuracy: ±2 bpm or ±2% (select larger) C. Resolution : 1%, Pulse rate: 1 bpm. D. Measurement Performance in Weak Filling Condition and pulse rate can be shown correctly when pulse-filling ratio is 0.4%.
-
Page 16: Installation
5 Installation 5.1 View of the Front Panel Figure 2. Front view 5.2 Battery Installation A. Refer to Figure 3. and insert the two AAA size batteries properly in the right direction. B. Replace the cover. Please take care when you insert the batteries for the improper insertion may damage the device. -
Page 17: Accessories
Figure 3. 5.3 Accessories A. a hanging rope B. a user manual C. a data line D. a disk (PC software) -
Page 18: Operating Guide
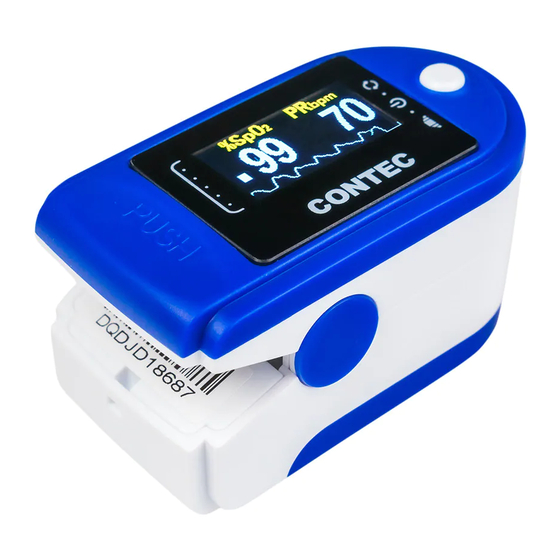
6 Operating Guide 6.1 Application Method a) Insert the two batteries properly to the direction, and then replace the cover. b) Open the clip as shown in Figure 4. Let the patient’s finger put into the rubber cushions of the clip (make sure the finger is in the right position), and then clip the finger. - Page 19 Figure 4. Put finger in position B. Change display direction The device could change display direction according to the handing direction. C. Pause alarm: a) Alarm including the alarm of measure data's going beyond the limits, the alarm of low-voltage, the alarm of finger's out of position.
- Page 20 program for receiving real-time data and stored data which is shown as Figure 5. a). Please connect the device to computer with the affiliated data line , then double click the "SpO Assistant"icon and click " " and then click the "Connect"to start the program. b).
- Page 21 high-low limits, data storage (recording). Please note in the Settings Menu: CLICK = short press of button and PRESS = prolonged push of button (1sec) Figure 6. Main Menu Interface a) Alarm setting On the main menu interface, click the button to select “Alarm”, Press the button (1sec) to enter the alarm setting interface as shown in Figure 7: Figure 7.
- Page 22 Figure 8. On the alarm direction setting interface,Click the button to select “SpO Alm”or “PR Alm”,then Press the button (1 sec) to enter the SpO or PR direction setting interface as shown in Figure 9: Figure 9. Click the button to select “Dir”, then Press the button to choose Up or Down (this will be the direction the value of the high-low limits of SpO and pulse rate will be adjusted)
- Page 23 ‘Down’, then Click the button to select high limit (High) or low limit (Low). Press the button and hold to adjust the selected limit to the desired lower value and release the button once the lower limit has been reached. If the alarm function is on, the device will provide medium-priority alarm signal when the data of SpO pulse rate is beyond the limit.
- Page 24 b) Data storage setting This instrument has the ability to store 24 hours worth of data. It can store the measured pulse rate and SpO value accurately, transfer the data to the computer, display the data and print reports. On the main menu interface as shown in Figure 7, Click the button to select “Record”, then press the button to choose whether store the data or not, choose “on”...
- Page 25 When the storage space is full, it displays “Memory is full” on the screen, and then shut down in a few seconds. But it will still display “Memory is full” by the next time you turn on the device on the purpose of warning the user, and a few seconds later enter the measuring interface.
-
Page 26: Attention For Operation
F. Standby mode If the measuring finger out of the device on the measuring interface, The product will enter standby mode when no signal is in the product within 5 seconds. The device can't being enter standby mode when it is in a state of storing. -
Page 27: Clinical Restrictions
H. Strenuous action of the subject or extreme electrosurgical interference may also affect the accuracy. Testee can not use enamel or other makeup. Please clean and disinfect the device after operating according to the User Manual(7.1). 6.3 Clinical Restrictions A. As the measure is taken on the basis of arteriole pulse, substantial pulsating blood flow of subject is required. - Page 28 judgement of anemic anoxia and toxic anoxia, some patients with serious anemia may also report good measurement.
-
Page 29: Maintain, Transportation And Storage25
7 Maintain, Transportation and Storage 7.1 Cleaning and Disinfecting Using medical alcohol to disinfect the device, nature dry or clean it with clean soft cloth. 7.2 Maintain A. Please clean and disinfect the device before using according to the User Manual(7.1). B. -
Page 30: Troubleshooting
8 Troubleshooting Trouble Possible Reason Solution 1. Place the finger properly and The SpO 1. The finger is try again. and Pulse properly 2. Try Rate can positioned. again, Go to a 2. The patient’s not be hospital for a displayed is too low to diagnosis... - Page 31 1. Please 1. The battery is change drained away batteries. almost drained 2. Please device away. Install the can not 2. The battery is battery again. be turned installed incorrectly. 3. Please 3. The device’s contact the malfunction. local service center.
-
Page 32: Key Of Symbols
9 Key of Symbols Signal Description Refer to instruction manual/booklet %SpO The pulse oxygen saturation(%) PRbpm Pulse rate (bpm) Low-voltage Open the alarm sound indication Open the pulse sound indication Menu button/Exit standby mode. Type BF Serial number 1. The finger clip falls off ( no finger inserted) 2. - Page 33 IP22 International Protection WEEE (2002/96/EC) European Representative This item is compliant with Medical Device Directive 93/42/EEC of June 14, 1993, a directive of the European Economic Community. Manufacturer Manufacture Date Storage Transport Temperature limitation Storage and Transport Humidity limitation Storage Transport Atmospheric pressure limitation...
- Page 34 This side up Fragile, handle with care Keep dry Recyclable...
-
Page 35: Function Specification
10 Function Specification Information Display Mode Pulse Oxygen 2-digit digital OLED display Saturation(SpO Pulse Rate(PR) 3-digit digital OLED display Pulse Intensity Bar-graph OLED display (bar-graph) Parameter Specification 0% ~ 100%, (the resolution is Measuring range 1%). 70% ~ 100%: ±2% ,Below Accuracy 70% unspecified. - Page 36 cycle. The deviation between average value and true value does not exceed 1% Safety Type Interior Battery, BF Type Pulse Intensity Continuous bar-graph display, Range the higher display indicate the stronger pulse. Battery Requirement 1.5 V (AAA size) alkaline batteries × 2 Battery working life Two1.5 V (AAA size) 600 mAh alkaline batteries can work continually for 24 hours...
- Page 37 Appendix1 Guidance and manufacturer’s declaration – electromagnetic emissions- for all EQUIPMENT and SYSTEMS Guidance and manufacturer’s declaration – electromagnetic emission The CMS50D+ intended for use in the electromagnetic environment specified below. The customer of the user of the CMS50D+ should assure that it is used in such and environment.
- Page 38 CMS50D+ suitable for use in all establishments other than domestic and those emission Class B directly connected to a CISPR voltage power supply network which supplies buildings used for domestic purposes. Guidance and manufacturer’s declaration – electromagnetic immunity – for all EQUIPMENT and SYSTEMS Guidance and manufacturer’s declaration –...
- Page 39 6 6 Electro Floors static contact contact should 8 kV air 8 kV air dischar wood, concrete (ESD) ceramic tile. If floor are 61000- covered with synthetic material, the relative humidity should be at least 30%. Power 3A/m 3A/m Mains power freque quality should...
- Page 40 NOTE is the a.c. mains voltage prior to application of the test level. Guidance and manufacturer’s declaration – electromagnetic immunity – for EQUIPMENT and SYSTEMS that are not LIFE-SUPPORTING Guidance and manufacturer’s declaration – electromagnetic immunity The CMS50D+ intended for use in the electromagnetic environment specified below.
- Page 41 Portable mobile communications equipment should be used no closer to any part of the CMS50D+, including cables, than Radi 3 V/m recommended ated separation distance calculated from the equation applicable to the frequency of 00-4 the transmitter. Recommended separation distance ...
- Page 42 80 MHz to 800 800 MHz to 2.5 Where P is the maximum output power rating of the transmitter in watts (W) according to transmitter manufacturer and d recommended separation distance in metres (m). Field strengths from fixed transmitters, determined by an...
- Page 43 electromagnetic site survey, should be less than the compliance level in each frequency range. Interference occur vicinity equipment marked with the following symbol: NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations.
- Page 44 predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the CMS50D+ is used exceeds the applicable RF compliance level above, the CMS50D+ should be observed to verify normal operation.
- Page 45 CMS50D+ intended electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the CMS50D+ can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the CMS50D+ as recommended below, according to the maximum output power of the communications equipment.
- Page 46 11.67 23.33 For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter watts according transmitter...
- Page 47 Appendix 2 State Alarm condition Alarm signal delay generation delay Low voltage 20ms alarm alarm 330ms 20ms Pulse rate 330ms 20ms alarm Probe error 16ms 20ms alarm...