Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents
Troubleshooting

Summary of Contents for Itamar Medical OM2196335
- Page 1 Operation Manual Itamar Medical REF OM2196335 Caution: Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner Copyright 2015-2020 By Itamar Medical Ltd. WatchPAT™ and PAT ® are trademarks of Itamar Medical, Ltd.
- Page 2 This manual and the information contained herein are confidential and are the sole property Itamar Medical Ltd. Only Itamar Medical Ltd. or its licensees have the right to use this information. Any unauthorized use, disclosure or reproduction is a direct violation of Itamar Medical’s proprietary rights.
- Page 3 Record of Editions Edition Date Description Chapter Pages ARMS – correcting typo (based Oct 2015 App.G OM2196331 Ed.6 and Ed.7) Jan 2016 Updating company logo Updating - Symbols Used on the 1.11 Product Labels, adding WEEE symbol June 2016 Updating Manufacturing App.F Declarations...
- Page 4 Jan 2018 Updating CE marking with the -,1.11, 1.12 i, 7-8 accompanying BSI notified body number 0086 Updating Intertek certification marks Deleting information in App H Adding zzzPAT HW -, App.H iii, 68 requirements and information regarding Manual March 2019 Updating CE marking with the -,1.11, 1.12 i,7-8...
-
Page 5: Table Of Contents
Table of Contents GENERAL INFORMATION ............. 1 Intended Use / Indications for Use ............1 Restrictions for Use ................1 Precautions ..................... 2 Data Generated by the WatchPAT™200U ..........2 Quality Assurance System: EN ISO 13485 ..........3 CSA Compliance ..................4 Conventions Used in this Manual ............ - Page 6 Replacing the uPAT Probe Cable ............31 Replacing the Battery ................32 Setting the Time and Date of the WatchPAT™ device ....... 33 Storing the WatchPAT™ device............33 APPLYING THE WATCHPAT™ DEVICE ......34 Preparing for Use of the WatchPAT™ Device ........34 Applying the WatchPAT™...
- Page 7 List of Figures Figure 1 – Packed Device ................... 11 Figure 2 – WatchPAT™200U Device with Sensors ........... 11 Figure 3 – The Buttons and Display ..............12 Figure 4 – Service Ports and Peripherals ............13 Figure 5 – WatchPAT™ Wrist Strap ..............13 Figure 6 –...
-
Page 8: General Information
Periodic review of the Manual is recommended. 10. Itamar Medical Ltd. makes no representation whatsoever, that the act of reading the Manual renders the reader qualified to operate, test or calibrate the system. -
Page 9: Precautions
12. In the event that the system does not operate properly, or if it fails to respond to the controls in the manner described in this Manual, the operator should refer to the Troubleshooting section. If necessary, contact our service office to report the incident, and to receive further instructions. -
Page 10: Quality Assurance System: En Iso 13485
1.5 Quality Assurance System: EN ISO 13485 The Itamar Medical WP200U is compliant to the following standards. STANDARD Medical electrical equipment – Part 1: General requirements IEC 60601-1 for basic safety and essential performance ANSI/AAMI ES60601 CAN/CSA -C22.2 No.60601-1 Medical electrical equipment – Part 1-2: General... -
Page 11: Csa Compliance
Requirements for regulatory purposes RoHS Directive 2011/65/EU (RoHS2) RoHS - Directive 2011/65/EU 1.6 CSA Compliance The product is certified by CSA. 1.7 Conventions Used in this Manual Note: Throughout this document, the references WatchPAT™, WatchPAT™200U and WP200U device are used to refer to the WatchPAT™200 Unified device. Note: Throughout this document, the reference Snore &... -
Page 12: Warnings, Cautions And Notes
Notes are used to identify an explanation, or to provide additional information for purposes of clarification. Les notes sont utilisées pour identifier les explications et pour donner des informations supplémentaires dans le but de clarifier. 1.8 Warnings, Cautions and Notes The WP200U is internally powered from a 4.2 V battery. -
Page 13: Safety Precautions
1.9 Safety Precautions WARNINGS Use only the AC adapter provided (5V DC, 5W maximum capacity power supply). Only authorized personnel may charge the WP200U. Failure to heed this warning may cause permanent damage to the equipment. Do not let the unit get wet. Avoid placing food or water on any part of the system. -
Page 14: Symbols Used On The Product Labels
1.10 Symbols Used on the Product Labels Follow instructions for use Type BF applied part The product is certified by CSA Date of manufacture YYYY-MM-DD 3.7V DC Battery Operating Voltage Single use, do not re-use Temperature limit Use-by date Medical device Manufacturer Catalogue Number Serial Number Ingress protection... -
Page 15: Watchpat™200U Device Labels
Caution: Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner According to the WEEE Directive 2012/19/EU, all waste electrical and electronic equipment (EEE) should be collected separately and not disposed of with regular household waste. Please dispose this product and all of its parts in a responsible and environmentally friendly way. -
Page 16: Overview
OVERVIEW Obstructive sleep apnea syndrome (OSAS) is considered a major public health problem. The prevalence of the syndrome is estimated at 2% to 5% in the adult population. It is characterized by recurrent events of complete or partial obstruction of the upper airways during sleep, often leading to hypoxemia, and/or arousals associated with sympathetic nervous system activation. -
Page 17: System Description
2.1 System Description The WP200U system is comprised of the following items: WP200U device that includes: o Embedded actigraph o Embedded pulse oximeter o Embedded CPU and electrical circuit card o Embedded micro SD card drive o Rechargeable Lithium Ion Battery o LCD display ... -
Page 18: Figure 1 - Packed Device
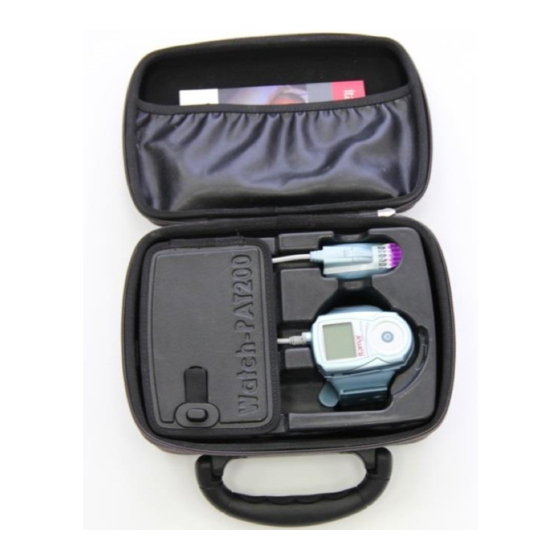
Figure 1 – Packed Device Optional SBP sensor Optional RESBP sensor uPAT probe Figure 2 – WatchPAT™200U Device with Sensors An additional item required for the operation of the system is the zzzPAT kit. zzzPAT is a proprietary PC software for initializing the study, retrieving, analyzing and displaying the data. -
Page 19: User Interaction With The Watchpat™ Device Keys
2.2 User Interaction with the WatchPAT™ Device Keys The WatchPAT™ has the following keys (see Figure 3): Central On/Enter key to power on the WatchPAT™ (the only key visible to the patient) Outer ring containing four keys (left, right, up, down) that may be used by the Operator for entering the diagnostic mode and navigating through the diagnostic menu. -
Page 20: Watchpat™ Device Function
The bracelet port is used for connecting the tamper-proof bracelet. The uPAT probe port is used for connecting the uPAT probe A port for connecting the optional Snore & Body Position sensor The USB port is used for charging or connecting to the PC uPAT probe port Bracelet port Port for optional Snore... -
Page 21: Built-In Self-Diagnostic Procedures
PAT ® Signal Oxygen saturation Actigraphy (movement) Acoustic decibel detector for Snoring evaluation (optional) Body Position (optional) The overnight sleep study data is stored on an embedded micro SD card in the WatchPAT™ device. After the study is recorded, the data is downloaded from the WatchPAT™... - Page 22 Press the ENTER button (Center key) for 2 seconds till the Itamar medical logo appears on the LCD screen Immediately press the UP + DOWN keys (see Figure 3) simultaneously for 1 second The following screen will be displayed:...
- Page 23 DEVICE TEST 22:50 ID=111-11-1111 pat=missing <-Back More-> TEST FAILED 2:54 The following are the possible error, warning or information messages: File error: not loaded, missing – the study file was not loaded or somehow the file was deleted File error: used x/3 x=1..3 – only when multi-night option is selected ...
- Page 24 If the WatchPAT™ device passes this self-diagnostic test, the following screen will be displayed: PATIENT 22:51 GOOD NIGHT!!! Time elapsed=9:50 Recording… Note During recording the LCD display turns off to conserve battery life. Any key pressed during Recording will turn on the LCD for 30 seconds. If the WatchPAT™...
- Page 25 Note The "x" stands for 0-F value (Hexadecimal code) Error codes are additive, i.e. both uPAT probe and File errors will produce error code xx6x. WatchPAT™200U System Operation Manual...
-
Page 26: Preparation For Sleep Study
3 PREPARATION FOR SLEEP STUDY Charging the Battery The battery must be charged every time the WatchPAT™ device is prepared for use. The battery may be charged using the AC adapter provided. To charge the WatchPAT™ device: Gently slide the WatchPAT™ device out of the wrist strap until a click is heard and the USB port is exposed. -
Page 27: Preparing The Snore And Body Position Sensor
The display will show “CHARGER” if you are charging with the AC adapter or “PC HOST” if you are charging with a computer. The current battery voltage is shown. Charge the battery the first time for approximately three hours. Thereafter recharging takes approximately 1-1.5 hours. -
Page 28: Mounting The Watchpat™ On The Wrist Strap
3.4 Mounting the WatchPAT™ on the Wrist Strap To mount the WatchPAT™ device on the wrist strap: Gently slide the WatchPAT™ device into the wrist strap until a click is heard indicating that it is properly seated. 3.5 Replacing the uPAT Probe Warning The uPAT probe connector is very sensitive and therefore should never be left exposed. -
Page 29: Preparing The Watchpat™ Device For A New Study
uPAT probe Snore and Body Position sensor Figure 9 – WatchPAT™ Fully Prepared 3.6 Preparing the WatchPAT™ Device for a New Study Refer to the zzzPAT Software Manual for preparation of the WP200U for a new study. 3.7 Testing the WatchPAT™ Device Run the built-in self-diagnostic facility as described in Section ... -
Page 30: Packing The Carrying Case
3.9 Packing the Carrying Case The following items must be placed inside the carrying case, in their respective compartments (see Figure 1 – Packed Device): The WatchPAT™ device mounted in the Wrist strap with the uPAT probe attached. Step-by-Step Reference Guide to the WatchPAT™ device. ... -
Page 31: Optional Functions
OPTIONAL FUNCTIONS 4.1 Using the integrated Snore & Body Position Sensor The integrated sensor consists internally of two sensors: a snore sensor and a body position sensor. A - Integrated sensor Sensor Attachment RESBP Sensor Attachment B - Integrated RESBP sensor The integrated sensor is powered by the WatchPAT™... -
Page 32: Tamper-Proof Testing With Watchpat™ Device
4.2 Tamper-Proof Testing with WatchPAT™ Device The WatchPAT™ device Tamper-Proof bracelet is an add-on accessory used to authenticate the patient doing a sleep study and assure the study is recorded from the right person. The bracelet is a single use small plastic band designed to be worn around the wrist of the hand. -
Page 33: Figure 12 - Watchpat™ Device With Cable For Bracelet
need to connect the Bracelet, via the bracelet's cable 2 connectors, to the WatchPAT™ device. The device will not start without connection to the paired Bracelet. Figure 12 – WatchPAT™ Device Figure 13 – WatchPAT™ Device with Cable for Bracelet with Bracelet Figure 14 –... -
Page 34: Multi-Night Study
4.3 Multi-night study A patient study may be defined as multi-night study and the patient can sleep up to 3 nights with the same WatchPAT™ device. The multi-night option may be selected during New Study function (see zzzPAT Operation Manual). If a 3 night multi-night option is selected the patient must replace the uPAT probe and charge the device between nights. -
Page 35: Data Download And Analysis
DATA DOWNLOAD AND ANALYSIS Following the sleep study the WatchPAT™ device is returned to the referring sleep clinic for data downloading and analysis by the zzzPAT software. To download and analyze the study data: 1. Connect the USB port of the WatchPAT™ device to the computer (see Figure 4) The WatchPAT™... -
Page 36: Maintenance
MAINTENANCE The WatchPAT™ device has been designed and manufactured to meet all safety requirements applicable to medical equipment. To ensure maximum safety of operation, the system should be used and maintained in strict compliance with the safety precautions, warnings and operating instructions provided in this Manual. In order to prevent unnecessary failures while patient is using the device, we recommend performing the routine maintenance recommendations as well as the preventive maintenance recommendations as described in this section. -
Page 37: Cleaning
Check strap Perform technician test Preventive maintenance recommendations: Scenario Routine maintenance Lesser of: 200 When a defect is action studies, 1 year, error found or upon error message in device message test Replace battery Replace PAT cable Replace SBP sensor Replace strap Replace charger Replace carrying case... -
Page 38: Handling
6.1.2 Cleaning the Wrist Strap You may clean the wrist strap with lint free cloth lightly moistened with 70% ethyl alcohol or isopropyl alcohol (IPA). In order to disinfect the wrist strap by immersing into disinfecting liquid follow the steps: Remove WatchPAT™... -
Page 39: Replacing The Battery
2. Connect a new uPAT probe cable by gently inserting the connector into the WatchPAT™ device. Make sure you secure back the screw. Figure 18 – Replacing the uPAT Probe Warning Use only the original screw that belongs to the WatchPAT™ device. Using different screw could harm the device. -
Page 40: Setting The Time And Date Of The Watchpat™ Device
4. Insert the new battery into the battery compartment. 5. Insert the 3 pin connector into the corresponding battery connector (one pin is longer so it may properly be inserted in only one direction). Secure the battery connector closed with a small piece of transparent tape. 6. -
Page 41: Applying The Watchpat™ Device
7 APPLYING THE WATCHPAT™ DEVICE Note These instructions are designed to help the patient use the WP200U after seeing a demonstration by trained personnel of how to mount the probes on his/her fingers and correctly operate the WatchPAT™ device. The following detailed instructions are summarized in the patient’s step-by-step reference guide. -
Page 42: Applying The Watchpat™ Device
7.2 Applying the WatchPAT™ Device To apply the WatchPAT™ device to your wrist: 1. Open the carrying case and take out the wrist strap with the WatchPAT™ device mounted. All parts should already be connected, as illustrated in Figure 9. 2. -
Page 43: Figure 23 - Placing Finger In Upat Probe
3. Detach and gradually remove the tab marked TOP slowly and firmly while pressing the tip of probe against a hard surface (WatchPAT™ case, table, etc.) until the tab is completely removed from the probe (Figure 24). You might feel a slight suction once the tab is removed. The uPAT probe is now attached (Figure 25). -
Page 44: Switching On The Watchpat™ Device
Note If the Snore & Body position sensor is included in the WatchPAT™ device case see Appendix A: WatchPAT™ Integrated snoring + Body Positioning Sensor Operating Instructions (SBP/RESBP) 7.4 Switching On the WatchPAT™ device You are now ready to switch on the WatchPAT™ device. Just before you lie down to go to sleep, firmly press the ON/Enter center button (Figure 3) until the LCD display lights up. -
Page 45: Important Notes
7.6 Important Notes Wearing the WatchPAT™ device should not cause any discomfort or pain. If you experience wrist or arm discomfort, loosen up the wrist strap. If the discomfort is not alleviated immediately, call the service number. Do not attempt to connect or disconnect any part of the unit. ... -
Page 46: Patient Training - Guidelines
8 PATIENT TRAINING – GUIDELINES Instruct the patients (and accompanying individual if needed) how to attach and use the WP200U prior to use. 8.1 Walk Through the Process of Using the WatchPAT™ device Product introduction – WatchPAT™ device, wrist strap, uPAT probe ... -
Page 47: Switching On The Watchpat™ Device
Secure the snoring sensor in place with medical tape. 3. Wearing the Wrist Strap Should be comfortable, not too tight. 4. Attaching the WatchPAT™ Device Make sure the WatchPAT™ device is properly mounted on the wrist strap. If it is loose, gently slide it in until you hear a click. -
Page 48: Troubleshooting Guide
9 TROUBLESHOOTING GUIDE 9.1 Operator Error Messages If an error message is displayed while performing the self-diagnostic tests, take the actions specified below. If the problem persists contact Itamar or an authorized representative. Table 1 – Operator Troubleshooting Error Possible Reason Action File error Not loaded... -
Page 49: Patient Error Messages
9.2 Patient Error Messages If an error message is displayed when the patient powers on the WatchPAT™ device, the patient should take the actions specified below. If the problem persists the patient may contact Itamar or an authorized representative directly. Table 2 –... -
Page 50: Specifications
10 SPECIFICATIONS Table 3 – WatchPAT™200U Specifications Properties Description Itamar’s proprietary probe. Measures PAT and uPAT Probe Oximetry. Recording Time Approx. 10 hours Channels Measuring 4-7 signals: PAT, Pulse rate, Oximetry, Actigraphy, Snoring (optional), Body Position (optional), Chest Movement (optional) PAT and Actigraph –... -
Page 51: Appendix A: Watchpat™ Integrated Snoring + Body Positioning Sensor Operating Instructions (Sbp/Resbp)
APPENDIX A: WatchPAT™ Integrated snoring + Body Positioning Sensor Operating Instructions (SBP/RESBP) SBP must be used with zzzPAT v 4.3 and above and WatchPAT™200/U RESBP must be used with zzzPAT v 4.6 and above and WatchPAT™200U with embedded 3.3228 and above Thank you for purchasing an Integrated Snore &... - Page 52 The snore sensor is an acoustic decibel detector. It uses a very sensitive microphone that responds to snoring and other sounds in the audio range and converts them to a small analog voltage that provides a clear, reliable indication of the presence of these sounds. The body position sensor uses a 3-axis accelerometer that provides a signal directly proportional to the patient's sleeping posture (supine, prone, right, left and sit).
- Page 53 Weight 12 gr Warranty 6 months Temperature Operation 0 to 40 Storage -20 to 40 Transport -20 to 60 0% – 93% (non- Humidity Operating, Storage & Transport condensing) 10 – 15 psi Atmospheric pressure Operating, & Storage 8 – 15 psi Transport WatchPAT™200U System Operation Manual...
- Page 54 Snoring and Body Position Accuracy This section gives statistical performance measure for Itamar SBP sensor, when used with the WatchPAT™ device. Body Position The body position measured by the WatchPAT™ device with Itamar SBP sensor was compared to the gold standard, manual scoring of the video recording of 31 patients, in 1 minute’s epochs (total of 7111 epochs) during sleep.
- Page 55 Coef. Of Lower Upper Mean Variation Median Value 2033 41.10 1.89 4.60 41.01 41.18 1319 41.61 2.67 6.43 41.47 41.76 42.68 3.79 8.88 42.44 42.93 44.12 4.49 10.19 43.80 44.44 44.75 4.65 10.39 44.41 45.09 45.90 5.07 11.04 45.51 46.30 46.45 5.17 11.13...
- Page 56 Summary statistics (mean ± SD) of WatchPAT™200U device by dB-meter levels Note The snoring and body position safety and effectiveness was validated on adult population only. WatchPAT™200U System Operation Manual...
-
Page 57: Appendix B: Tamper-Proof Testing With Watchpat
APPENDIX B: Tamper-proof testing with WatchPAT™ WatchPAT™200U System Operation Manual... - Page 58 WatchPAT™200U System Operation Manual...
-
Page 59: Appendix C: License Agreement
This License Agreement is a legal agreement between you (as an individual, company, organization or other entity) and Itamar Medical Ltd. (“Itamar”). By installing, copying, or otherwise using the Licensed Software, and/or by using the Licensed Product or third party product into which a Licensed Product or Licensed Software is incorporated (“Third Party... - Page 60 incorporated as a component within such Third Party Product. 1.2. “Licensed Software” means the zzzPAT software, the associated media and accompanying materials provided to you with such zzzPAT software. Some Licensed Software is a stand-alone product and some Licensed Software is incorporated as a component within a Licensed Product, in each case sold or otherwise made available, by Itamar and/or third parties.
- Page 61 copyright, trademark, logo or other proprietary marking or legend placed on or contained in the Licensed Software or a Licensed Product. 4. LIMITED WARRANTIES AND DISCLAIMERS a. Against Infringement. Itamar hereby warrants to you that it has the right to grant you the license to use the Licensed Software and/or the Licensed Product and to enter into this License Agreement and that neither the Licensed Software nor the Licensed Product(s) infringes the intellectual property rights of any third party.
- Page 62 While every reasonable effort has been made to ensure that you will receive Licensed Software that you can use, Itamar does not warrant that the functions of the Licensed Software will meet your requirements or that the operation of the Licensed Software will be uninterrupted or error free.
- Page 63 NO INDEMNIFICATION OBLIGATIONS WITH RESPECT TO ANY INFRINGEMENT CLAIM TO THE EXTENT ARISING FROM YOUR USE OF THE LICENSED PRODUCT AND/OR LICENSED SOFTWARE IN CONJUNCTION WITH OTHER HARDWARE OR SOFTWARE WHERE USE WITH SUCH OTHER HARDWARE OR SOFTWARE GAVE RISE TO THE INFRINGEMENT CLAIM. 6.
- Page 64 Note: Should you have any questions concerning this License Agreement, or if you desire to contact Itamar for any reason, please write to: USA: Itamar Medical Inc. 3290 Cumberland Club Drive, Suite 100 Atlanta, Georgia 30339, USA Tel: 1 888 748 2627 Worldwide: Itamar Medical Ltd.
-
Page 65: Appendix D: Regulatory Representative
APPENDIX D: REGULATORY REPRESENTATIVE Itamar Medical’s authorized regulatory representative is: Arazy Group GmbH The Squaire 12, Am Flughafen, 60549 Frankfurt am Main, Germany WatchPAT™200U System Operation Manual... -
Page 66: Appendix E: Description Of The Watchpat™200U Upat Probe
APPENDIX E: DESCRIPTION OF THE WATCHPAT™200U UPAT PROBE The WatchPAT uPAT probe is an opto-pneumatic finger-mounted probe. Its role is to continuously measure the relative state of the vasomotor activity in the distal part of the finger based on a plethysmographic method. The uPAT probe is designed to cover the distal part of the finger with a uniform pressure field extending to the tip of the finger. -
Page 67: Appendix F: Manufacturing Declarations According
The recommended separation distances in this paragraph must therefore be complied with. • The Itamar Medical’s WP200U must not be used near or on top of another device. If this cannot be avoided, it is necessary – before clinical use – to check the equipment for correct operation under the conditions of use. - Page 68 Table 2 - based on IEC 60601-1-2:2014 Guidance and manufacturer's declaration – electromagnetic immunity – WP200U The WP 200U is intended for use in the electromagnetic environment specified below; The customer or the user of the WP200U should assure that it is used in such an environment. IEC 60601-1-2 Immunity test Compliance level...
- Page 69 determined by an electromagnetic site survey , should be less than t he compliance level in each frequency range . Interference may occur in the vicinity of equipment marked with the following symbol: NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations .Electromagnetic propagation is affected by absorption And reflection from structures objects and people.
- Page 70 Recommended Separation Distances The Itamar Medical’s WP200U is intended for use in an electromagnetic environment in which radiated radiofrequency disturbances are controlled. The user and/or installer of the unit can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile radiofrequency communications equipment (emitters) and the Itamar Medical’s WP200U, according to the maximum output power of the...
- Page 71 Table 9 - based on IEC 60601-1-2:2014 Proximity Fields from RF Wireless Communication Equipment Test Band Service Modulation Maximum Distance Immunity Minimum frequency power (W) TEST separation (MHz) (MHz) LEVEL distance (V/m) 360-390 TETRA 400 Pulse modulation 18 Hz 430-470 GMRS 460, FRS 460 ±5 kHz...
- Page 72 Appendix G: SpO2 accuracy in the WATCHPAT™200U The WatchPAT™200U device uses Itamar Medical Pulse Oximetry system for the measurement of functional oxygen saturation of arterial haemoglobin (SpO2). This appendix includes information regarding the accuracy of these measurements following a clinical study of Itamar Medical Pulse Oximetry.
- Page 73 WatchPAT™200U System Operation Manual...
- Page 74 2013-08-20 Clinical Investigator(s): Clinimark 80 Health Park Drive, Suite 20 Louisville, Colorado 80027, USA Sponsor: Itamar Medical, Ltd. 9 Halamish St POB 3579, Caesarea 3088900 Israel Device(s): Non-Motion: Itamar Medical WatchPAT 200 Pulse Oximetry Study Date(s): May 8-10, 2013 Note A Functional tester cannot be used to assess the accuracy of the internal pulse oximeter.
-
Page 75: Appendix H: Zzzpat Hardware Requirements
APPENDIX H: ZZZPAT HARDWARE REQUIREMENTS Hardware configuration: Computer Pentium 4 3GHz or higher 1 available USB port XGA screen resolution (minimum 1024 x 768 pixels) Colors set to 16 bits or higher RAM 1GB or higher Disk space requirements: Standalone installation o 10GB minimum / 60GB recommended disk space on Files folder and at least 1.2GB on boot drive ... -
Page 76: Appendix I: Spare Parts List
APPENDIX I: SPARE PARTS LIST The following items can be ordered and purchased individually: uPAT probe (a box of 12 uPAT probes) uPAT probe connection cable Wrist Strap Snore and Body Position sensor (SBP) Snore and Body Position sensor (RESBP) ...










Need help?
Do you have a question about the OM2196335 and is the answer not in the manual?
Questions and answers