Table of Contents
Advertisement
Quick Links
TM
Endo PAT
2000 Device
User Manual
Itamar Medical REF OM1695214
This product and/or method of use, is covered by one or more of the following US patents: 6319205,
6322515, 6461305, 6488633, 6916289, 6939304, 7374540, as well as any pending US patent
applications and corresponding patents and/or applications filed in other countries.
TM
, EndoScore™ and PAT™ are trademarks of Itamar Medical, Ltd.
Endo PAT
Caution: Federal (U.S.) law restricts this
device to sale by, or on the order of, a physician.
Copyright 2002-2015 by Itamar Medical Ltd.
Software Version 3.7.x
Advertisement
Table of Contents
Troubleshooting

Summary of Contents for Itamar Medical EndoPAT 2000
-
Page 1: User Manual
6322515, 6461305, 6488633, 6916289, 6939304, 7374540, as well as any pending US patent applications and corresponding patents and/or applications filed in other countries. , EndoScore™ and PAT™ are trademarks of Itamar Medical, Ltd. Endo PAT Caution: Federal (U.S.) law restricts this device to sale by, or on the order of, a physician. - Page 2 SUCH LICENSE AGREEMENT IS PROHIBITED. DISCLAIMER Itamar Medical Ltd. shall not be held responsible in any manner for any bodily injury and/or property damage arising from operation or use of this device other than that which adheres to the instructions and safety precautions contained herein and in all supplements hereto and according to the terms of the warranty provided in Appendix A.
- Page 3 Removing an erroneous restriction entered in version 20 March 14 Adding trade-mark symbols and relevant updated MEDES address updating App B, 10.4 70, 78 Itamar Medical address updating - zip code, and Ind. Park -,10.4 i, 70 May 2014 Insert note Updating Endo PAT™2000 label System 10.4...
- Page 4 Itamar Medical Ltd. Feb 2015 Updating Caution and Warning symbols Updating Manufacturing Declarations App.E 91-94 Adding spare parts list App.F Place the device on a stable platform Tube placement while storing Warning – advise the use of anti-virus SW and backups Adding French translation 1.7, 1.8, 3.4,...
-
Page 5: Table Of Contents
Itamar Medical Ltd. Table of Contents General Information .............. 1 2000 Device .......... 1 Intended Use of the Endo PAT Performance and clinical study information ..........1 Equipment Classification ................ 3 Manufacturers Notice ................3 Restrictions for Use ................3 Quality Assurance System: ISO 9001 &... - Page 6 Itamar Medical Ltd. Connecting the PAT™ Probe ..............35 Creating a Patient File ................36 2000 Study ........ 41 Conducting an Endo PAT Pre-Study ..................... 41 Patient and System Setup..............43 Performing the Study ................45 Review and Analysis ............51 Study Data Retrieval ................
- Page 7 Itamar Medical Ltd. Appendix A: License Agreement and Limited Warranty ....79 Appendix B: Regulatory Authorized Representative ....... 85 Appendix C: Installing the USB adaptor for Windows XP ....86 Appendix D: Installing the USB adapter for Windows 7 or 8 ... 92 APPENDIX E: MANUFACTURING DECLARATIONS ACCORDING TO IEC 60601-1-2 ...........
- Page 8 Itamar Medical Ltd. Figure 21 - The setup dialog box (full application and viewer mode) ......30 Figure 22 – Report Setup dialog box ................32 Figure 23 - The example for report header ..............32 Figure 24 - The PATographer Information dialog box ..........33 Figure 25 - COM port search ..................
- Page 9 Itamar Medical Ltd. Figure 53 - MOXA USB Installation – XP2 ..............87 Figure 54 - MOXA USB Installation – XP3 ..............87 Figure 55 - MOXA Adapter ..................88 Figure 56 - MOXA Adapter Configuration – XP2 ............88 Figure 57 - MOXA Adapter Configuration –...
-
Page 10: General Information
Itamar Medical Ltd. General Information This manual is part of the Endo PAT 2000 system. Intended Use of the Endo PAT 2000 Device The Endo PAT 2000 device is a non-invasive device, intended for use as a diagnostic aid in the detection of coronary artery Endothelial Dysfunction (positive or negative) using a reactive hyperemia procedure. - Page 11 Itamar Medical Ltd. analysis system. Average peak velocity (APV) is derived from the Doppler flow velocity spectra and coronary blood flow (CBF) is determined as: *(coronary artery diameter/2) *(APV/2). Endothelium-dependent coronary flow reserve is calculated as percent change in CBF in response to the Ach challenge.
-
Page 12: Equipment Classification
Manufacturers Notice The information in this document is subject to change without notice. Itamar Medical Ltd. makes no warranty of any kind on this material, including but not limited to, the implied warranties of merchantability and fitness for a particular purpose. -
Page 13: Quality Assurance System: Iso 9001 & Iso 13485
The device is not intended as a screening test in the general patient population. Itamar Medical Ltd. makes no representation whatsoever, that the act of reading this User Manual renders the reader qualified to operate, test or calibrate the system. - Page 14 Itamar Medical Ltd. Medical devices. Symbols to be used with medical device ISO 15223-1 labels, labelling and information to be supplied. General requirements Graphical symbols for electrical equipment in medical IEC TR 60878 practice Graphical symbols -- Safety colours and safety signs -- ISO 7010-M002 Registered safety signs;...
-
Page 15: Conventions Used In This Manual
Itamar Medical Ltd. Conventions Used in this Manual The following conventions are used throughout this manual: Warnings Are used to identify conditions or actions, which - if the instructions are ignored - may violate patient safety, or could cause damage/malfunction of the system, resulting in the irretrievable loss of data. -
Page 16: Safety Precautions
The system contains no user-serviceable parts. It should be maintained and serviced only by qualified service personnel, authorized by Itamar Medical Ltd. Purchasers of the Endo PAT 2000 device should ensure that only suitably trained, qualified personnel are authorized to operate the equipment. - Page 17 Itamar Medical Ltd. authorized operators, and stored where it is easily accessible. Periodic review of the manual is recommended. The Endo PAT 2000 system is a whole system. To eliminate risk of electrical shock, do not attempt to open or remove system covers or plugs.
-
Page 18: System Overview
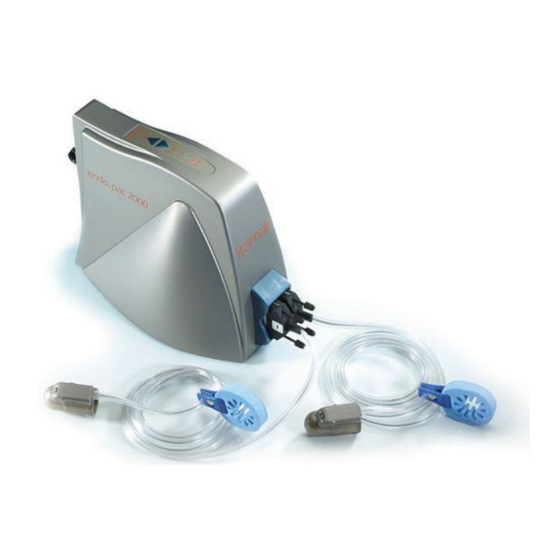
Itamar Medical Ltd. System Overview The Endo PAT 2000 device is a computer-based system for non-invasively assessing ™ vascular endothelial dysfunction. It is based on the use of Peripheral Arterial Tone (PAT signal technology, during a clinically established procedure, which measures post-ischemic vascular responsiveness following upper arm blood flow occlusion. -
Page 19: Installing The System
Itamar Medical Ltd. Installing the System Basic System Configuration The Endo PAT 2000 device is supplied as a complete package comprising the following components: One Endo PAT 2000 device One Endo PAT 2000 software CD One HASP (dongle) required for full application only ... -
Page 20: System Description
Itamar Medical Ltd. System description The Endo PAT 2000 device top panel has: Power LED indicator LED indicator for the device-computer communication status Probe’s Deflate and Inflate buttons Power LED Indicator Power Supply DC Connector Communication LED Indicator... -
Page 21: Connecting The Endo Pat
Itamar Medical Ltd. Connecting the Endo PAT 2000 device to the Computer NOTE The Endo PAT 2000 system requires the use of a serial (RS232) port in the computer with a standard 9-pin RS232 cable. The Endo PAT 2000 device can alternatively be connected through a USB to RS232 adapter (supplied with the system) 1. -
Page 22: Endo Pat Tm 2000 Software Installation
Itamar Medical Ltd. Endo PAT 2000 Software Installation NOTE Prior to software installation, verify that you are in full system administrator mode with full privileges. Otherwise, the installation might not succeed and could cause operational problems. NOTE When installing the full software application (versions 3.7 and up) make sure you have an Itamar-Medical... -
Page 23: Figure 4 - Installshield Wizard
Itamar Medical Ltd. 3. The Installshield wizard prepares the computer for installation. When prompted, click next to proceed with the installation (Figure 4). Figure 4 - Installshield wizard 4. Read the license agreement and select the “I accept” option to agree to license terms. -
Page 24: Figure 6 - Installation Option
Itamar Medical Ltd. Figure 6 – installation option 6. Click “Next” to set the default target folder for software installation, or click “Change” to select a different folder for the installation (Figure 7). Figure 7 - Installation folder selection NOTE It is not recommended to install the program in the “My Documents”... -
Page 25: Figure 8 - Ready To Install The Program Screen
Itamar Medical Ltd. 7. Press “Install” to complete the installation process or “Back” to review or change any of your installation settings” (Figure 8). Figure 8 – Ready to install the program screen 8. Press “Finish” when the installation is completed (Figure 9). -
Page 26: Installing The Rs-232 To Usb Adaptor
Itamar Medical Ltd. WARNING The Endo PAT 2000 device is a PC operator device. Use antivirus software to protect your system and files. You should also consider routine backups to ensure your ability for data recovery if needed. Avertissement L'appareil EndoPAT 2000 est utilisé... -
Page 27: Registration
Registration enables you to benefit from our special offers when available, and to be able to activate some of our newer features. Registration enables Itamar Medical to notify you when new version of your product is available and helps Itamar Medical provide you with customer support. -
Page 28: Figure 10 - Registration Reminder And Number Of Left Trial Recording
Itamar Medical Ltd. Figure 10 - Registration reminder and number of left trial recording Note Your license has expiration date. The system will notify in time to renew your license. Use the registration process to renew your license. To register the software installation you first need to create a registration request file. Use the following instructions to create registration file and register your installation: 3.6.1.1 Open the registration window: from the “PAT™... -
Page 29: Figure 12 - Registration Dialog
3.6.1.4 If you wish to enable any of the additional / research features, check the feature you want to activate. The use of these features is limited, and they will be opened subject to Itamar Medical’s approval. NOTE AI, FRHI and HRV have not been submitted to the US Food and... -
Page 30: Figure 13 - Registration Request File Instructions
Once you obtain your EndoHASPRegistration.req file, send it to lic@itamar-medical.com or contact your local distributor. Itamar Medical will receive your request, process it and you will receive the EndoFullLicense.lic file by e-mail. Place the license file in your installation directory and restart the Endo PAT™ application. -
Page 31: Figure 14 Viewer Mode Registration
3.6.2.4 If you wish to enable any of the additional / research features, check the feature you want to activate. The use of these features is limited, and they will be opened subject to Itamar Medical’s approval. NOTE AI, FRHI and HRV have not been submitted to the US Food and... -
Page 32: Uninstalling Endo Pat
Once you obtain your EndoRegistration.req file, send it to lic@itamar-medical.com contact your local distributor. Itamar Medical will receive your request, process it and you will receive the License.lic file by e-mail. Place the license file in your installation directory and restart the Endo PAT™ application. -
Page 33: Shutting Down The System
Itamar Medical Ltd. NOTE Make sure to keep your HASP after uninstalling the full system. Your HASP will be used in the registration of your new version. Shutting Down the System a. Shut down the Endo PAT 2000 software program by selecting Exit in the pull down File menu. -
Page 34: Software Description
Itamar Medical Ltd. Software Description The following screens describe the full application. Viewer mode has the relevant subset of the controls (the title of the screen might be different). Main Screen From the Windows desktop double click the icon. The following screen will appear (see Figure 16). -
Page 35: Figure 17 - Fill Site Name Dialog Box
Itamar Medical Ltd. Tool bar (section 4 .3) Scroll bar (section 4 .3.2) 2. Display windows: Channels identification column (for the PAT™ waveforms) PAT™ waveforms window Results/calculations column 3. Status bar: PAT™ state (communication status between PAT™ device and computer) ... -
Page 36: Main Screen Menu Commands
Open Batch Analysis dialog box Help Provides access to system information Link to Itamar Medical Uploading Service Table 1 - Main screen pull down menu commands Main Screen Tool Bar The Main Screen tool bar buttons provide quick access to selected menu commands, opens result table, and to the Gain and Timing settings. -
Page 37: Table 2 - Tool Bar Buttons And Functions
Itamar Medical Ltd. Table 2 lists each button and its function. “Mouse over” a button to trigger bubble help describing the button’s function. Button Function Load file Print screen Open Patient Information Dialog Box Deflate PAT™ probes Inflate PAT™ probes... -
Page 38: Configuring The System
Itamar Medical Ltd. 4.3.1 Gain and Time-base trace display Tools Use the Gain command, to adjust the Trace Window display. Figure 19 - Gain and time-base scroll boxes The two gain tools adjust the traces’ display of the PAT™ 1 and PAT™ 2 channels (The scroll boxes are in order from left to right: left is probe1 and right is probe2). -
Page 39: Figure 20 - The Setup Command
Itamar Medical Ltd. 2. Click PAT™ Control, and then select Setup. Figure 20 - The setup command The following screen will appear: Figure 21 - The setup dialog box (full application and viewer mode) 3. Click “Automatic Search (COM1-COM10)” to allow the system to automatically identify the COM port to which the Endo PAT 2000 device is connected. - Page 40 Itamar Medical Ltd. 6. Select the EndoScore™ index that will be used. The options are the LnRHI or the previous index- the RHI. See section 7 .4 for more information about the 2 indices. 7. In the “Cardiovascular Risk Factors” frame enter the default method for calculating risk factor to be used in the system.
-
Page 41: Figure 22 - Report Setup Dialog Box
Itamar Medical Ltd. Figure 22 – Report Setup dialog box Figure 23 - The example for report header Endo PAT 2000 Device Operation Manual... -
Page 42: Figure 24 - The Patographer Information Dialog Box
Itamar Medical Ltd. 16. The name of the operator performing the Endo PAT 2000 study can be saved with the study data. The system offers the user to choose from a predefined list, or enter names manually, if the manually entered name doesn’t exist in the list it will be automatically added to the list of offered names. -
Page 43: Using The Timer (Countdown Clock) (Full Application Only)
Itamar Medical Ltd. NOTE The default inflation pressure setting for the PAT™ Sensors is 50 mmHg in “fixed” mode. It is recommended that this is not exceeded, unless specified otherwise. (full application only) NOTE Setup can be opened while recording a study, however, during a recording the COM field and the Pressure Control fields are disabled and cannot be modified. -
Page 44: Preparing For A Study
Itamar Medical Ltd. Preparing for a Study Preparing the System for a Study Accessories that are required beside the Endo PAT 2000 system: A set of two PAT probes and anchors Blood pressure cuff (capable of sustaining high pressures for 5 minutes) ... -
Page 45: Creating A Patient File
Itamar Medical Ltd. Figure 26 - Inserting into slit Figure 27 - Clicking in To remove probes, press the tab (clip) marked by the arrow in Figure 28, and then lift the connector away from the probe (Figure 29). Used probes should be disposed of properly. -
Page 46: Figure 30 - Patient Information Dialog Box (Metric Version)
Itamar Medical Ltd. Visit – Enter visit number or code. Up to 9 characters. This field should be used to distinguish between several tests by the same patient (with the same ID). The patient ID and visit are used to generate the file name used by the system. -
Page 47: Figure 31 - "File Id Exists" Warning Message
Itamar Medical Ltd. Risk Factors… - this will open a dialog box about additional inputs required to calculate Cardio-vascular Risk Factor. See paragraph 3 below. “New Patient” button will clear the dialog and allow filling the dialog in ... - Page 48 Itamar Medical Ltd. calculations see section 7 .5. There are 3 possible methods for calculating the Cardiovascular Risk: Framingham Risk Score, SCORE Risk and Reynolds Risk Score. The default method is set in the setup (see 4 .4), but it can be changed ...
-
Page 49: Figure 32 - Patient Information - Risk Factors
Itamar Medical Ltd. (HTN) Gender Taken from the patient information main dialog box Systolic blood pressure Table 3 – Risk Factors mandatory fields Gray lines represent inputs that are used in the calculation of the Risk, but are part of the main Patient Information Dialog and not the Risk Factors Dialog. -
Page 50: Conducting An Endo Pat 2000 Study
Itamar Medical Ltd. Conducting an Endo PAT 2000 Study Pre-Study 6.1.1 General description The Endo PAT system is comprised of a system console and two independent sensing probes coupled to connecting pneumo-electric tubing and foam finger mounting rings. The system console is connected to a computer loaded with a specific program for controlling the Endo PAT system. - Page 51 Itamar Medical Ltd. The patient should then be comfortably seated or allowed to lie down in the study room and relax for at least 15 minutes or a sufficient period to reach a relaxed cardiovascular steady-state and to adjust to the room temperature.
-
Page 52: Patient And System Setup
Itamar Medical Ltd. Patient and System Setup 6.2.1 Study conditions The study should be conducted in a quiet and relaxed atmosphere. Phones, beepers and other devices which can cause startling noises should be turned off; otherwise the startle effect on the patient might affect the test result. The patient should be kept comfortable and fully relaxed and asked to refrain from talking. -
Page 53: Figure 34 - Applying The Pat™ Probes
Itamar Medical Ltd. 6.2.3 Patient preparation First, ensure that a blood pressure cuff is placed on the upper arm of the designated test arm. Then, the PAT™ probes should be placed inside the appropriate sockets of the arm- supports (see Figure 34-1). Fully deflate the probes by clicking the icon in the software or by pressing the “Deflate”... -
Page 54: Performing The Study
Itamar Medical Ltd. The patient should be instructed to refrain from moving the fingers to the extent possible. Both patients’ forearms should be supported on the arm supports (alternatively, rolled towels or bed-sheets can be used). Make sure that the probes are free and not in contact with any object (including the supporting surface), as shown in Figure 35. -
Page 55: Figure 36 - Standby Mode
Itamar Medical Ltd. NOTE Newer system will include a 20 seconds ‘flatten’ signal periods that will alternate between the probes. This is done to emphasis the system is in standby mode. Continues signals will resume once the recording starts. NOTE If you are in the Stand By mode, it is possible to stop the test, deflate &... -
Page 56: Figure 37 - Recording
Itamar Medical Ltd. NOTE While working in the Stand By mode, data is not recorded, and therefore it is impossible to review or save the study. A notification about this mode (Figure 36) is displayed, and will disappear when the button is clicked. - Page 57 Itamar Medical Ltd. NOTE After starting the recording the time scale will be set automatically so the full window will contain 1 minute. 15 seconds periods will be marked by dotted lines. If the beginning of the recording is marked by patient motion artifacts or an unstable signal, consider troubleshooting procedures or extend the total period of baseline recording to give an overall period of at least 5 minutes of stable baseline data prior to the occlusion.
-
Page 58: Figure 38 - Occlusion Quality Assessment
Itamar Medical Ltd. Warning Inflating the BP cuff might cause some stress and discomfort to the patient. Pay attention to the patient’s well-being. Avertissement Le gonflement du brassard BP risque de mettre le patient dans un état de stress et de gêne. Soyez attentifs au confort du patient. - Page 59 ID as the file name. By default, the data folder is located in the data folder, in the Itamar Medical folder in C drive. It can also be accessed directly from the desktop by using the icon.
-
Page 60: Review And Analysis
Itamar Medical Ltd. Review and Analysis During a PAT™ study, recorded signals are viewed in the display window and, based on the appearance of the traces, a qualitative evaluation can be performed. However, subsequent review of the study using the special features described in this chapter facilitates a quantitative analysis of the acquired data. -
Page 61: Figure 40 - Automatic Analysis
Itamar Medical Ltd. Figure 40 - Automatic analysis NOTE The AI (Augmentation Index) is one of the additional / Research parameters, and it is calculated and presented only if enabled as part of the license. The automatic analysis identifies the occlusion borders and marks with two vertical blue lines its beginning and end, and also marks blue the whole signals area between these two lines. -
Page 62: Figure 41 - Occlusion Popup Menu
Itamar Medical Ltd. The RHI (Reactive Hyperemia Index) or LnRHI (natural log of RHI) is the post-to-pre occlusion PAT™ signal ratio in the occluded arm, relative to the same ratio in the control arm, corrected for baseline vascular tone of the occluded arm where: Normal EndoScore™:... -
Page 63: Study Report
Itamar Medical Ltd. NOTE It is recommended to change the time-base to a 1 minute screen (00:01:00) to make the identification of the occlusion borders easier. If the occlusion area extends beyond the edge of the window, the window will automatically scroll as you drag the mouse across its edge. -
Page 64: Figure 42 - Non Selective Population Histograms Of Rhi
Itamar Medical Ltd. Endo PAT 2000 study results 7.4.1 EndoScore™ result: RHI and LnRHI The RHI (Reactive Hyperemia Index) is the post-to-pre occlusion PAT™ signal ratio in the occluded arm, relative to the same ratio in the control arm, corrected for baseline vascular... -
Page 65: Figure 43 - Non Selective Population Histograms Of Lnrhi
Itamar Medical Ltd. Figure 43 - non selective population histograms of LnRHI The LnRHI distribution curve in a non-selected population is included in the report (Figure 44) and displays the LnRHI result in respect to non selected population. Figure 44 – LnRHI distribution in non-selective population The graph presents the distribution function of the LnRHI in the nonselected population. -
Page 66: Cardiovascular Risk
Itamar Medical Ltd. 7.4.3 Patient Historical results If the report setup allows for historical result to be presented, and there are previous EndoScore™ result for the current patient ID that meets the criteria and deviation range (as set in the report setup) the report will include a historical result section containing a table and a chart. -
Page 67: Additional / Research Features
Itamar Medical Ltd. Those with a strong family history of premature CVD The socially deprived Subject with diabetes – risk may be 5 fold higher in women with diabetes and 3 fold higher in men with diabetes compared to those without diabetes. -
Page 68: Figure 46 - Ai Calculation
Itamar Medical Ltd. NOTE To activate any Additional / Research Feature, you should submit a request to Itamar Medical as part of the Registration process. See section 3 .6 above. 7.6.1 Augmentation Index (AI) Augmentation index is a measure of arterial stiffness, calculated based on pulse wave analysis of the signal measured by the Endo PAT™... -
Page 69: Figure 47 - Ai Result From The Report (Female Example)
Itamar Medical Ltd. Figure 47 – AI result from the report (female example) 7.6.2 Heart rate variability (HRV) Heart rate variability (HRV) is a measure of heart beat to beat variability in either time or frequency domain. HRV reflects the status of the autonomic nervous system (ANS) and particularly the balance between sympathetic and parasympathetic activities. -
Page 70: Tabular Report
(1.5- 2minutes) than the RHI/LnRHI. Researchers that want to use this index for research purposes can ask Itamar Medical to enable this index as part of the registration process. The FRHI will be added to the tabular report (see Table 4). - Page 71 Itamar Medical Ltd. The table lists relevant study parameters and results, for all analyses performed to date, with the last line in the table containing data from the most recent analysis performed. Table 4 is a description of the information fields displayed in the table.
-
Page 72: Table 4 - Table Information
Itamar Medical Ltd. FileName visit EndoScore™ RHI: Reactive Hyperemia Index ( result) EndoScore™ LnRHI: Natural Base log of Reactive Hyperemia Index ( result) BL HR: baseline heart-rate AI: Augmentation Index AI@75: Augmentation Index - normalized to HR 75bpm AI_N pulses: number of pulses averaged to calculate the AI... -
Page 73: Batch Analysis
Itamar Medical Ltd. Batch Analysis The Endo PAT 2000 software allows the user to perform a batch automatic analysis on a group of studies as follows: The batch analysis command analyzes all the files located in a selected folder. If necessary, copy the files you wish to analyze to a new folder before proceeding. -
Page 74: Figure 50 - Marking Segments And Artifacts
Itamar Medical Ltd. NOTE While marking segments and artifacts, errors may be corrected by clicking icon (“Clear all segments”). This will also erase the occlusion border markings. This tool should be used only when using the manual options described in this chapter. - Page 75 Itamar Medical Ltd. Select a segment and click icon to mark it as the B segment - B segment traces are highlighted in green. Select a segment and click on the icon to mark it as a T segment - T segment traces are highlighted in red.
-
Page 76: 7.10 Study Printing
7.11 Uploading data to the server The software offers a quick link to the Itamar Medical Uploading Service: from the Help menu, click on “Link to Itamar Medical Uploading Service”. Follow the instruction in the browser to upload files. This function requires a connection to the internet. The software will open the default browser with the correct link. -
Page 77: Maintenance
2000 system. Only qualified medical personnel should use this equipment. In the event of equipment malfunction all repairs should be executed by qualified Itamar Medical personnel or authorized service agents. Maintenance instructions should be followed closely to avoid unnecessary equipment failure or potential health hazards to the user or patient. -
Page 78: Troubleshooting
Itamar Medical Ltd. Figure 51 - Probes Information If a probe is missing or cannot be read, NA will appear instead of probe’s information. Troubleshooting Description Possible Cause Action Switch on the Endo PAT 2000 device. The Endo PAT... -
Page 79: Table 5 - Troubleshooting
Itamar Medical Ltd. Description Possible Cause Action Follow instructions provided with the USB to USB to Serial adapter not Serial adapter to verify proper installation. installed, or installation did not complete properly. Verify that the pneumatic probe cable is... - Page 80 Some critical analysis parameters were Please contact customer support. changed in an unauthorized way. To The device can record but not analyze prevent unexpected results, running the data. analysis is blocked. Contact Itamar Medical for more help. Endo PAT 2000 Device Operation Manual...
-
Page 81: Table 6 - Troubleshooting License
EndoPAT program will be closed. Please connect your dongle and try again. License violation detected. Please The system detected a major discrepancy, renew license and contact customer please contact Itamar Medical support. immediately. Table 6 - Troubleshooting license Endo PAT 2000 Device... -
Page 82: Table 7 - Error Messages
Explanation Unable to open file (-n) The system cannot open the file. The code in parenthesis (n) provides additional information for technical support (call Itamar Medical customer support). Signal Length Less Than The recorded signal length is less than the minimum required to run an Minimum Required analysis (6 min). -
Page 83: Technical Information
Itamar Medical Ltd. Technical Information 10.1 System Minimum Requirements ® An IBM or compatible PC Pentium/Celeron/AMD 1000 MHz CPU or higher Any Internet browser or Excel 2000 and above 1 GB RAM for XP or 2 GB for Win7 / Win8... - Page 84 Itamar Medical Ltd. The product complies with the CE mark according to MDD (Medical Device Directive) and related standards. The product is marked with the CE logo. Use-by date Single use, do not re-use Temperature limit 40ºC 0ºC Medical device Manufacturer...
-
Page 85: 10.4 Labeling
Itamar Medical Ltd. 10.4 Labeling Label on the base of the Endo PAT 2000 device (main control unit), older device on the left and devices from revision 6 and up on the right: Packaging labels: The following labels are attached to the master package of the Endo PAT... - Page 86 Itamar Medical Ltd. Endo PAT 2000 Probe Package: Endo PAT Pneumatic PAT Probe Medes Ltd. Itamar Medical Ltd. 5 Beaumont Gate, Shenley Hill, 9 Halamish St, P.O.Box 3579 Radlett, HertfordshireWD7 7AR. Caesarea Ind. Park, 3088900, Israel England Tel: + 972 4 6177000...
-
Page 87: Tm 2000 System
Itamar Medical Ltd. 10.5 Specifications for Endo PAT 2000 system Properties Description Itamar Medical’s proprietary probe only PAT™ Probe Recording Time Limited by hard disk space, ~8MB per study of 20 minutes Sampling Resolution 12 bit 2 LED’s - power supply and communication Indications PAT™... -
Page 88: Appendix A: License Agreement And Limited Warranty
This License Agreement is a legal agreement between you (as an individual, company, organization or other entity) and Itamar Medical Ltd. (“Itamar”). By installing, copying, or otherwise using the Licensed Software, and/or by using the Licensed Product or any Third Party Product, you agree to be bound by the terms of this License Agreement with respect to the Licensed Software and Licensed Products. - Page 89 Itamar Medical Ltd. Products are incorporated as components within Third Party Products, in each case sold or otherwise made available, by Itamar and/or third parties. If you have received this License Agreement with a Third Party Product, this License Agreement applies only to the Licensed Product incorporated as a component within such Third Party Product.
- Page 90 Itamar Medical Ltd. regulatory agency of any diagnostic or therapeutic claim, or medical device, pharmaceutical or other therapeutic or diagnostic product. Without derogating from the generality of the foregoing, the inclusion by you or any third party of any results of any...
- Page 91 Itamar Medical Ltd. b. As to Licensed Product. Itamar warrants that the Licensed Product, with which this License Agreement was delivered, will be free from defects in design, materials, and workmanship for a period of one year from the date of delivery of the Licensed Product to you.
- Page 92 Itamar Medical Ltd. MERCHANTABILITY, FITNESS PARTICULAR PURPOSE, ACCURACY COMPLETENESS INFORMATION, LACK NEGLIGENCE AND CORRESPONDENCE TO DESCRIPTION. LIMITATION OF LIABILITY TO THE MAXIMUM EXTENT PERMITTED BY APPLICABLE LAW, EXCEPT FOR DAMAGES ARISING UNDER SECTION 4(A) ABOVE, IN NO EVENT SHALL ITAMAR BE LIABLE TO YOU FOR DAMAGES IN EXCESS OF THE PURCHASE PRICE YOU PAID FOR THE LICENSED SOFTWARE, THE LICENSED PRODUCT OR THE APPLICABLE THIRD PARTY PRODUCT.
- Page 93 Note: Should you have any questions concerning this License Agreement, or if you desire to contact Itamar for any reason, please write to: Itamar Medical Ltd., 9 Halamish St., Caesarea Ind. Park, 3088900, Israel, Facsimile: +972-4-627 5598, or visit Itamar’s web site at www.itamar-medical.com.
-
Page 94: Appendix B: Regulatory Authorized Representative
Itamar Medical Ltd. Appendix B: Regulatory Authorized Representative MEDES Ltd. 5 Beaumont Gate, Shenley Hill, Radlett, Hertfordshire WD7 7AR. England Tel: +44 20 8123 8056 Tel / Fax: +44 1923859810 Endo PAT 2000 Device Operation Manual... -
Page 95: Appendix C: Installing The Usb Adaptor For Windows Xp
Itamar Medical Ltd. Appendix C: Installing the USB adaptor for Windows XP This appendix describes how to install the MOXA adapter and driver for Windows XP Home and for Windows XP Pro editions NOTE The MOXA adapter must not be connected to the computer or to the Endo PAT 2000 device while the driver is installed. -
Page 96: Figure 2 - Endo Pat Tm 2000 Device
Itamar Medical Ltd. 1.6 Click “Install”. 1.7 The following screen is displayed: Figure 53 - MOXA USB Installation – XP2 1.8 Click “Continue Anyway” (you need to approve this warning twice). The following screen is displayed: Figure 54 - MOXA USB Installation – XP3 1.9 Click “Finish”. -
Page 97: Figure 55 - Moxa Adapter
Itamar Medical Ltd. 2. Configuring the MOXA Adapter 2.1 Plug in the adapter to your USB port. Figure 55 - MOXA Adapter 2.2 Wait for the following window to appear. Figure 56 - MOXA Adapter Configuration – XP2 2.3 Select the “No, not this time” option, and click the “Next” button. -
Page 98: Figure 57 - Moxa Adapter Configuration - Xp3
Itamar Medical Ltd. Figure 57 - MOXA Adapter Configuration – XP3 2.4 Select the “Install the software automatically (Recommended)” option, then click the “Next” button. The following window appears: Figure 58 - MOXA Adapter Configuration – XP4 2.5 Wait for the installation wizard to find the UPort 1110 driver; then, click the “Next”... -
Page 99: Figure 59 - Moxa Adapter Configuration - Xp5
Itamar Medical Ltd. Figure 59 - MOXA Adapter Configuration – XP5 2.6 Click the “Continue Anyway” button. The following window is displayed: Figure 60 - MOXA Adapter Configuration – XP6 2.7 Click the “Finish” button. 2.8 Repeat steps 2-7 again when the “Welcome to the found new hardware wizard”... -
Page 100: Figure 61- Connect Moxa Adaptor
Itamar Medical Ltd. 3. Connecting the adapter to the Endo PAT 2000 device 3.1 Connect the MOXA Adapter to the COM TO COM cable and tightly screw the bolts. Figure 61- connect MOXA adaptor 3.2 Connect the COM TO COM cable to the Endo PAT 2000 device and tightly screw the bolts. -
Page 101: Appendix D: Installing The Usb Adapter For Windows 7 Or 8
Itamar Medical Ltd. Appendix D: Installing the USB adapter for Windows 7 or 8 This appendix describes how to install the MOXA adapter and driver for Win7 and Win8. NOTE The MOXA adapter must not be connected to the computer or to the Endo PAT 2000 device while the driver is installed. -
Page 102: Figure 64 - Win7 Security
Itamar Medical Ltd. Figure 64 - Win7 Security 1.3 When the following window will open, press the Next Button. Figure 65 - MOXA Uport driver installation 1.4 The following window will open, press the Next button. Endo PAT 2000 Device... -
Page 103: Figure 66 - Moxa Driver Installation Folder
Itamar Medical Ltd. Figure 66 - MOXA driver installation folder 1.5 The following window will open, press the Install button. Figure 67 - MOXA driver folder confirmation 1.6 Press the Finish button Endo PAT 2000 Device Operation Manual... -
Page 104: Figure 68 - Moxa Driver Installation Finish
Itamar Medical Ltd. Figure 68 - MOXA driver installation finish 2. Configuring the MOXA Adapter 2.1 Plug in the adapter to your USB port. Figure 69 - The MOXA Adapter 2.2 The following icon should appear at the window notification area zone while windows installs the driver needed for the MOXA adapter (this is done automatically). - Page 105 Itamar Medical Ltd. 2.3 When the installation of the driver is done the following message should appear: Endo PAT 2000 Device Operation Manual...
-
Page 106: Figure 70- Connect Moxa Adaptor
Itamar Medical Ltd. 3. Connecting the adapter to the Endo PAT 2000 device 3.1 Connect the MOXA Adapter to the COM TO COM cable and tightly screw the bolts. Figure 70- connect MOXA adaptor 3.2 Connect the COM TO COM cable to the Endo PAT 2000 device and tightly screw the bolts. -
Page 107: Appendix E: Manufacturing Declarations According To Iec 60601-1-2
- before clinical use – to check the equipment for correct operation under the conditions of use. The use of accessories other than those specified or sold by Itamar Medical as replacement parts may have the consequence of increasing the emissions or decreasing the immunity of the unit. - Page 108 Itamar Medical Ltd. Table 2 - from IEC 60601-1-2:2007 Guidance and manufacturer's declaration – electromagnetic immunity – Endo PAT 2000 (ED2000) The ED2000 is intended for use in the electromagnetic environment specified below; The customer or the user of the ED2000 should assure that it is used in such an environment.
- Page 109 Itamar Medical Ltd. Table 4 - from IEC 60601-1-2:2007 Guidance and manufacturer's declaration – electromagnetic immunity – Endo PAT 2000 (ED2000) The ED2000 is intended for use in the electromagnetic environment specified below; The customer or the user of the ED2000 should assure that it is used in such an environment.
-
Page 110: Recommended Separation Distances
Itamar Medical Ltd. Recommended Separation Distances The Endo PAT 2000 is intended for use in an electromagnetic environment in which radiated radiofrequency disturbances are controlled. The user and/or installer of the unit can help prevent electromagnetic interference by maintaining a... -
Page 111: Appendix F: Spare Parts List
Itamar Medical Ltd. APPENDIX F: SPARE PARTS LIST The following items can be ordered and purchased individually: Pneumatic PAT Probe (a box of 12 probes) Pneumo-electric tubing Power adaptor Power Cable USB adaptor RS 232 cable ...











Need help?
Do you have a question about the EndoPAT 2000 and is the answer not in the manual?
Questions and answers
how to calculate LnRHI ?
The LnRHI (Natural Log Reactive Hyperemia Index) for the Itamar Medical EndoPAT 2000 is a natural log transformation of the RHI (Reactive Hyperemia Index). The formula to calculate LnRHI is:
LnRHI = ln(RHI)
where ln represents the natural logarithm function.
The RHI itself is calculated as the post-to-pre occlusion PAT™ signal ratio in the occluded arm, relative to the same ratio in the control arm, corrected for baseline vascular tone.
This transformation does not change the normal/abnormal classification but provides a distribution closer to a normal (Gaussian) distribution.
This answer is automatically generated