Table of Contents
Advertisement
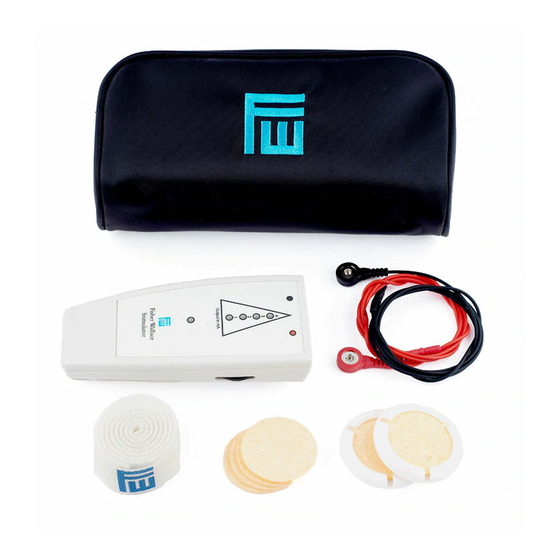
THE FISHER WALLACE STIMULATOR
FOR THE TREATMENT OF PAIN ON THE BODY
INSTRUCTION
MANUAL. Call us with any questions: (800) 692 - 4380
1.0 INTRODUCTION
The Fisher Wallace Stimulator is a portable, battery-powered device which has been cleared by the FDA for
symptomatic relief of chronic pain, post-traumatic acute pain and post-surgical pain when used on the body. The
device is also cleared by the FDA for the treatment of depression, anxiety and insomnia. Although the device
poses no significant risks to health, patients should be monitored by their physician at least once every three
months while using the device; more frequent monitoring is recommended. Do not attempt to reduce or cease
use of other medication without first consulting your doctor.
The device delivers a micro-electrical stimulus (1-4 mA) that is carried by electrical cables to wet sponges (supplied
with the device) that are applied to the skin. When treating site-specific pain on the body, the electrodes are
placed on the body. As clearly illustrated in this manual on pages 6-12, the red wire electrode is typically placed at
the rear base of the neck and the black wire electrode is placed on the pain site (with the exception of gastro pain
treatment). The electrodes are secured to the body with Velcro body straps, which are included in the kit.
Read section 10.0 APPLICATION OF THE DEVICE ON THE BODY for instructions for use, as well
as the Treatment Diagrams on pages 6-12 for guidance regarding the treatment of Back Pain, Shoulder
Pain, Gastro Pain, Elbow Pain, Phantom Limb Pain, Arthritic Pain and Neck Pain.
Please email us at info@fisherwallace.com or call us at (800) 692 - 4380 with any questions.
2.0 CONTRAINDICATIONS
The device is contraindicated for use in patients who have demand or sensing type cardiac pacemakers.
3.0 WARNINGS
Keep the device out of the reach of children. Electronic monitoring equipment (such as ECG monitors, ECG
alarms) may not operate properly when CES stimulation is in use. High Frequency surgical equipment may not be
used when CES stimulation is in use. Do not operate the CES stimulator in close proximity to short-wave or
microwave therapy equipment.
This device is not suitable for use with oxygen or in the presence of a flammable anesthetic mixture with air or
oxygen, or with nitrous oxide.
4.0 PRECAUTIONS
This device should not be used on the throat or neck, except the rear base of the neck as indicated in the diagrams
for treating pain (see the Chronic Pain Instruction Manual). This device should not be used on the eyes. This
device should not be used on or near areas of the body, including the head, that contain implanted devices, such as
stimulators, stents, and active or inactive implants such as deep brain stimulators and vagus nerve stimulators.
Patients should avoid using the device near areas of the body where there is embedded shrapnel or metal plates.
There is no danger in using the device if you have dental fillings.
Do not allow water to enter this device. The Fisher Wallace Stimulator should not be exposed to environmental
conditions where the system may get wet.
5.0 ADVERSE REACTIONS (VERY RARE)
Skin irritation may occur at the site of sponge electrode placement, especially if the sponges deteriorate (sponges
should be replaced EVERY TWO WEEKS) or if contact is made between the skin and the metal part of the
sponge receptacle, or if the sponges are not thoroughly wet before each use. A mild headache or dizziness may
occur, and should cease after you stop using the device. Such reactions are very rare.
PL-‐1003
R ev.
A
1
Advertisement
Table of Contents

Summary of Contents for FW Fisher Wallace Stimulator
- Page 1 Patients should avoid using the device near areas of the body where there is embedded shrapnel or metal plates. There is no danger in using the device if you have dental fillings. Do not allow water to enter this device. The Fisher Wallace Stimulator should not be exposed to environmental conditions where the system may get wet.
- Page 2 7.0 INDICATIONS FOR USE The Fisher Wallace Stimulator is a portable battery powered pulse generator that may be used for symptomatic relief of chronic pain, post-traumatic acute pain and post-surgical pain. Your physician will prescribe the appropriate duration to utilize the Fisher Wallace Stimulator.
- Page 3 Make sure the sponges are wet. Use the enclosed Velcro body straps to secure the red wire electrode to the rear base of the neck (see below) and the black wire electrode to the area of the body that is experiencing pain as indicated in the diagrams.
- Page 4 with alcohol and wipe down the base unit. Once clean, reinsert the wires into the base unit, matching the wire colors to the circles on the base unit. Now you can continue to use the device. 11.0 BATTERY REPLACEMENT In order to replace the battery, remove the battery compartment cover on the back of the device by sliding the cover into the open position.
- Page 5 15.0 LIMITED 1-YEAR WARRANTY Fisher Wallace Laboratories warrants each new Fisher Wallace Stimulator (exclusive of batteries) to be free from defects in materials and workmanship for a period of one (1) year following the delivery of the device to the original purchaser. The obligation of Fisher Wallace Laboratories under this warranty is expressly limited solely and exclusively to the repair or replacement of the unit or any parts thereof, which to Fisher Wallace’s satisfaction, shall have become defective during the...
- Page 6 This warranty shall not apply to any Fisher Wallace Stimulator which has been repaired, tampered with or altered by someone other than a Fisher Wallace Laboratories representative or technician, or which has been subjected to negligence, accident, mishandling or which has not been used in accordance with the enclosed instructions or for the stated purposes.
- Page 7 Yellow Light Indicator/ Amperage Level: 3 or 4 PL-‐1003 R ev. A ...
- Page 8 Yellow Light Indicator/ Amperage Level: 3 or 4 PL-‐1003 R ev. A ...
- Page 9 Yellow Light Indicator / Amperage Level: 3 or 4 PL-‐1003 R ev. A ...
- Page 10 Yellow Light Indicator/ Amperage Level: 3 or 4 PL-‐1003 R ev. A ...
- Page 11 Yellow Light Indicator/ Amperage Level: 3 or 4 PL-‐1003 R ev. A ...
- Page 12 Yellow Light Indicator/ Amperage Level: 3 or 4 IF NECK PAIN RADIATES TO BOTH SIDES OF THE UPPER BACK, USE THE BLACK LEAD SEPARATELY ON EACH SIDE FOR 10-20 MINUTES. PL-‐1003 R ev. A ...
- Page 13 Yellow Light Indicator/ Amperage Level: 3 or 4 PL-‐1003 R ev. A ...
Need help?
Do you have a question about the Fisher Wallace Stimulator and is the answer not in the manual?
Questions and answers